Lactic Acid Fermentation vs. Alcoholic Fermentation
... Lactic acid fermentation is a biological process by which glucose and other six-carbon sugars (also, disaccharides of six-carbon sugars, e.g. sucrose or lactose) are converted into cellular energy and the metabolite lactate. There are several uses of this type of fermentation, one of them to produce ...
... Lactic acid fermentation is a biological process by which glucose and other six-carbon sugars (also, disaccharides of six-carbon sugars, e.g. sucrose or lactose) are converted into cellular energy and the metabolite lactate. There are several uses of this type of fermentation, one of them to produce ...
ATP ENERGY PRODUCTION
... coupling of actin and myosin protein filaments = protein filaments cant attach to each other. The sliding of the muscle protein filaments = not possible. ...
... coupling of actin and myosin protein filaments = protein filaments cant attach to each other. The sliding of the muscle protein filaments = not possible. ...
Elements Found in Living Things
... 5. When polymers are built, the process is called a dehydration reaction. Why is the word “dehydration used?” Water is removed to form the bond between monomers 6. Organisms not only build molecules, they also break them down. This chemical reaction is called Hydrolysis 7. What does the prefix “hyd ...
... 5. When polymers are built, the process is called a dehydration reaction. Why is the word “dehydration used?” Water is removed to form the bond between monomers 6. Organisms not only build molecules, they also break them down. This chemical reaction is called Hydrolysis 7. What does the prefix “hyd ...
Citric Acid Cycle
... In the very next step, the 5-carbon moiety is again oxidatively decarboxylated with the formation of another NADH and another CO2. ...
... In the very next step, the 5-carbon moiety is again oxidatively decarboxylated with the formation of another NADH and another CO2. ...
The ketogenic diet
... the liver converts fat into fatty acids and ketone bodies. The ketone bodies pass into the brain and replace glucose as an energy source. ...
... the liver converts fat into fatty acids and ketone bodies. The ketone bodies pass into the brain and replace glucose as an energy source. ...
Muscle Activity Objectives SKELETAL MUSCLE ACTIVITY Definitions
... • A high energy phosphate is removed from CP and joined to ADP (adenosine diphosphate) to form ATP • Last for about 20 secs ...
... • A high energy phosphate is removed from CP and joined to ADP (adenosine diphosphate) to form ATP • Last for about 20 secs ...
Word
... A) It oxidizes succinate to fumarate B) It directly transfers electrons to cytochrome c. C) It reduces succinate to malate D) It is positively regulated by a high FADH2/FAD ratio E) The NADH it produces is used to reduce Coenzyme Q10 16) Which choice (A — E) for filling in the blanks makes a true st ...
... A) It oxidizes succinate to fumarate B) It directly transfers electrons to cytochrome c. C) It reduces succinate to malate D) It is positively regulated by a high FADH2/FAD ratio E) The NADH it produces is used to reduce Coenzyme Q10 16) Which choice (A — E) for filling in the blanks makes a true st ...
Chemistry SL HL Assessment Statements 2009 Revised
... The aim of this option is to give students an understanding of the chemistry of important molecules found in the human body, and the need for a balanced and healthy diet. Although the role that these molecules play in the body should be appreciated, the emphasis is placed on their chemistry, and stu ...
... The aim of this option is to give students an understanding of the chemistry of important molecules found in the human body, and the need for a balanced and healthy diet. Although the role that these molecules play in the body should be appreciated, the emphasis is placed on their chemistry, and stu ...
Name 1 Bio 451 12th November, 1999 EXAM III This
... B. In muscle, glutamine synthetase (GS) is very active, catalyzing the ATP-dependent formation of glutamine from glutamate and ammonia. In liver, GS activity is very low; however, the activity of glutaminase, which catalyzes the hydrolysis of glutamine to ammonia and glutamate, is high. Explain the ...
... B. In muscle, glutamine synthetase (GS) is very active, catalyzing the ATP-dependent formation of glutamine from glutamate and ammonia. In liver, GS activity is very low; however, the activity of glutaminase, which catalyzes the hydrolysis of glutamine to ammonia and glutamate, is high. Explain the ...
Chapter 4 The Importance of High
... -reactions with small, positive ΔG value are often part of important metabolic pathway in which they are followed by reactions with large negative ΔG value -single reaction never occurs independently, rather the nature of the equilibrium is constantly being changed through the addition and removal o ...
... -reactions with small, positive ΔG value are often part of important metabolic pathway in which they are followed by reactions with large negative ΔG value -single reaction never occurs independently, rather the nature of the equilibrium is constantly being changed through the addition and removal o ...
Chapter 4 The Importance of High
... -How is the protein synthesis (having positive ΔG value of 0.5 kcal/mole for each peptide bond) possible thermodynamically? -Biosynthesis is almost always coupled with energy consumption (소모) of negative ΔG (e.g., hydrolysis of ATP) adenosine-O-P~P~P + H2O Æ adenosine-O-P~P + P (ΔG = -7kcal/mole) ad ...
... -How is the protein synthesis (having positive ΔG value of 0.5 kcal/mole for each peptide bond) possible thermodynamically? -Biosynthesis is almost always coupled with energy consumption (소모) of negative ΔG (e.g., hydrolysis of ATP) adenosine-O-P~P~P + H2O Æ adenosine-O-P~P + P (ΔG = -7kcal/mole) ad ...
Transport and Metabolism Group work
... o The catabolic processes that you would find being used by these bacteria (summarized in Part A). o Note that enzymes are carrying out these metabolic reactions! o How energy is stored during these processes ATP • Substrate-level phosphorylation and/or • Oxidative phosphorylation Proton motive ...
... o The catabolic processes that you would find being used by these bacteria (summarized in Part A). o Note that enzymes are carrying out these metabolic reactions! o How energy is stored during these processes ATP • Substrate-level phosphorylation and/or • Oxidative phosphorylation Proton motive ...
Chapter 19
... • Odd carbon fatty acid oxidation produces propionyl-CoA, which is converted to succinylCoA. In the conversion, B12 cofactor enzyme, methylmalonyl-CoA mutase rearranges the carbon skeleton. • Excess of acetyl-CoA is converted to ketone bodies (acetoacetate + β-hydroxybutyrate) in mitochondria of liv ...
... • Odd carbon fatty acid oxidation produces propionyl-CoA, which is converted to succinylCoA. In the conversion, B12 cofactor enzyme, methylmalonyl-CoA mutase rearranges the carbon skeleton. • Excess of acetyl-CoA is converted to ketone bodies (acetoacetate + β-hydroxybutyrate) in mitochondria of liv ...
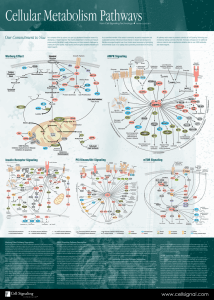
Cellular Metabolism Pathways
... Cancer cells frequently use glutamine as a secondary fuel source, which enters the mitochondria and can be used to replenish Krebs Cycle intermediates or can be used to produce more pyruvate through the action of malic enzyme. Highly proliferative cells need to produce excess lipid, nucleotide, and ...
... Cancer cells frequently use glutamine as a secondary fuel source, which enters the mitochondria and can be used to replenish Krebs Cycle intermediates or can be used to produce more pyruvate through the action of malic enzyme. Highly proliferative cells need to produce excess lipid, nucleotide, and ...
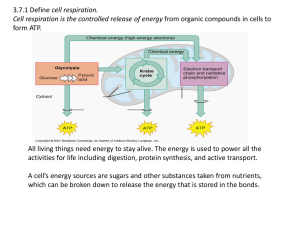
3.7:Cell Respiration Aerobic cell respiration: glucose
... IB Question: Compare anaerobic cellular respiration and aerobic cellular respiration. [5] Direct comparisons must be made to achieve a mark. anaerobic in the absence of oxygen whereas aerobic in the presence of oxygen; both may produce 2 CO ; both produce ATP; aerobic releases considerably more ATP ...
... IB Question: Compare anaerobic cellular respiration and aerobic cellular respiration. [5] Direct comparisons must be made to achieve a mark. anaerobic in the absence of oxygen whereas aerobic in the presence of oxygen; both may produce 2 CO ; both produce ATP; aerobic releases considerably more ATP ...
APB Chapter 9 Cellular Respiration: Harvesting Chemical Energy
... During glycolysis, glucose, a six-carbon sugar, is split _____________________________________________. ...
... During glycolysis, glucose, a six-carbon sugar, is split _____________________________________________. ...
fuels and tissues
... Can adapt to use of ketone bodies during fast (note: long chain FA cannot cross blood brain barrier and cannot be used as fuel by brain) but still require carbohydrates. KB may account for as much as 60% of fuel after prolonged fast. ...
... Can adapt to use of ketone bodies during fast (note: long chain FA cannot cross blood brain barrier and cannot be used as fuel by brain) but still require carbohydrates. KB may account for as much as 60% of fuel after prolonged fast. ...
Fall Semester Review - mychandlerschools.org
... Egg Rolling and the Greylag Goose If one of the gooses' egg rolls away from the nest, the goose automatically rolls the egg back to the nest with a repeated, specific action. When the female notices an egg outside the nest (sign stimulus), she begins this repeated movement to drag the egg with her ...
... Egg Rolling and the Greylag Goose If one of the gooses' egg rolls away from the nest, the goose automatically rolls the egg back to the nest with a repeated, specific action. When the female notices an egg outside the nest (sign stimulus), she begins this repeated movement to drag the egg with her ...
REGISTERED DIETITIAN EXAMINATION
... 7. The function of Vitamin E in humans includes (a) the synthesis of glycoproteins (b) prevention of oxidation in cell membranes (c) maintenance of Calcium phosphate balance (d) aiding reproduction ...
... 7. The function of Vitamin E in humans includes (a) the synthesis of glycoproteins (b) prevention of oxidation in cell membranes (c) maintenance of Calcium phosphate balance (d) aiding reproduction ...
Biology 231
... quaternary structure – some proteins are composed of more than 1 polypeptide chain held together like tertiary structures enzymes – 100s of protein catalysts (end in –ase) function depends on structure very specific – only catalyze specific reactions substrate – reactant molecule(s) enzyme acts on ...
... quaternary structure – some proteins are composed of more than 1 polypeptide chain held together like tertiary structures enzymes – 100s of protein catalysts (end in –ase) function depends on structure very specific – only catalyze specific reactions substrate – reactant molecule(s) enzyme acts on ...
PhotosynthesisCalving CycleON
... twice in order to make a molecule of glucose. (Actually 6 times). 1. Carbon dioxide combines with ribulose biphosphate. Ru-Bp is a pentose monosacharide with 2 ...
... twice in order to make a molecule of glucose. (Actually 6 times). 1. Carbon dioxide combines with ribulose biphosphate. Ru-Bp is a pentose monosacharide with 2 ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑