* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 4 The Importance of High
Amino acid synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Proteolysis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biosynthesis wikipedia , lookup
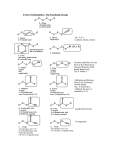
Chapter 4 The Importance of High-Energy Bond High energy bond: covalent bond Food for thought: does the biosynthetic processes violate (위반하다) thermodynamics (열역학)? Seemingly (겉으로 보기에) yes, but actually (실제로) not Æ input of high-energy activated (활성화된) precursors (전구체) Molecules that donate energy are thermodynamically unstable -The weaker the covalent bonds, the larger the amount of free energy, and vice versa Æ the stronger the bond, the less energy it can give off (내어놓다) -What is the best food in terms of chemical sense: molecules that can readily (쉽게) donate energy, contain weak covalent bonds (e.g., glucose) and are therefore thermodynamically unstable (compare CO2 and glucose, 어느 것이 더 좋은 음식, 또는 에너지원 인가? 좋은 음식의 조건, 우리는 화학에너지를 먹고 산다!!) -CO 2 Æ glucose in plant: need input of light energy Æ results in the formation of ATP -even a weak covalent bond is very strong Æ need energy supply to break Æ achieve (달성하다) activation state -activation energy is usually less than the original bond energy, because molecular rearrangements do not involve the production of completely (완벽히) free atoms -activation energy ranges 20-30 kcal/mole Æ not spontaneous at physiological temperature Æ preventing spontaneous rearrangement of cellular covalent bond -all these features are important for the existence (생존) of life (A-B) + (C-D) Æ (A-D) + (C-B) Keq = concA-D x concC-B / concA-B x concC-D ΔG = -RT ln Keq or Keq = e-ΔG/RT Figure 1. The energy of activation Enzymes lower activation energies in biochemical reactions -enzymes: essential for life, biocatalyst (생체촉매) -how function: speed up the rate of chemical reaction by lowering activation energy -enzymes can make it achieve to the lowest free energy status (상태) of reactants without affecting (영향을 미치다) the nature of equilibrium (평형) Figure 2 Free energy in biomolecules -unstable food molecules are destined (운명짓다) to convert into more stable and smaller molecules (e.g., CO2, water) through degradative (붕괴적인) pathway -Two purposes of degradative pathway (oxidative pathway) 1) produce small organic fragments (조각) necessary as building block 2) conserve (보존하다) the free energy (e.g., ATP: 세포내의 기축통화?) -Food molecule Æ free energy (40 % of glucose) + heat + entropy High-energy bonds hydrolyze with large negative ΔG -average high energy bond: ~ 7 kcal/mole -free energy stored in one glucose molecule is 688 kcal/mole Æ need multiple step to completely degrade (붕괴시키다) the molecule -Kinds of high-energy bonds: all involve P or S atoms -ATP is the most important P type -acetyl-CoA is the most important S-type Æ main source of fatty acid biosynthesis High-energy bonds in biosynthetic reaction -construction/deconstruction vs biosynthesis/degradation -biosynthetic pathway demand (요구하다) an external (외부의) source (원천) of free energy -thus, coupled with the breakdown of a high energy bond -but not all steps of biosynthesis require the breakdown of a high energy bond Figure 3. multistep metabolic pathway Peptide bonds hydrolyze spontaneously -ΔG for the formation of dipeptide = 1 ~ 4 kcal/mole Æ not spontaneous -but take into account the fact that the concentration of water molecule much higher than any other cellular molecules ( >>100 times) Amino acid(A) + amino acid (B) Æ dipeptide (A-B) +H2O Keq = concA-B x concH2O /concA x concB ΔG = -RT ln Keq -relative (상대적인) concentration is very important -in theory, proteins are unstable and will spontaneously degrade to free AA -But this process is too slow to affect cellular mechanisms in the absence of specific enzymes -Thus proteins remain (유지되다) stable unless (~하지 않으면) their degradation is not catalyzed by specific enzymes Coupling of negative with positive ΔG -How is the protein synthesis (having positive ΔG value of 0.5 kcal/mole for each peptide bond) possible thermodynamically? -Biosynthesis is almost always coupled with energy consumption (소모) of negative ΔG (e.g., hydrolysis of ATP) adenosine-O-P~P~P + H2O Æ adenosine-O-P~P + P (ΔG = -7kcal/mole) adenosine-O-P~P~P + H2O Æ adenosine-O-P + P~P (ΔG = -8kcal/mole) adenosine-O-P~P + H2O Æ adenosine-O-P + P (ΔG = -6kcal/mole) -Peptide bond formation is coupled with the breakdown of ATP to AMP and PP Activation of precursors in group transfer reaction -energy Æ heat or chemical bonds in coupled reactions, useful when coupled or useless (소용없는) when not coupled -coupled reaction is achieved by two or more successive (연속적인) reactions Æ group transfer reaction, in which always involves molecular exchange (교환) of functional groups (A-X) + (B-Y) Æ (A-B) + (X-Y) : group transfer (A-B) + (H-OH) Æ (A-OH) + (B-H) : hydrolysis -group transfer involves activated molecules associated with high-energy bonds Æ group activation ATP + GMP Æ ADP + GDP ATP + GDP Æ ADP + GTP ATP versatility (다용도임) in group transfer -ATP synthesis: a key in the controlled energy trapping in oxidative phosphorylation & photosynthesis (ADP + P + energy Æ ATP) -ATP is the original biological recipient of high-energy groups Æ must be the starting point for thermodynamically favorable (선호하는) reactions -contains two high-energy bonds -high-energy quality retains (유지되다) only when transferred (전달하다) to an appropriate (적당한) acceptor (수용체) molecules (e.g., COO~P, -C-O~P) Figure 4 Activation of amino acids by attachment of AMP -activation of amino acids are required (필요로하다) for the protein synthesis -these coupled reactions involves (~을 수반하다) the removal of activating group and conversion (전환) of a high-energy bond into one with a lower free energy of hydrolysis AA + ATP Æ AA~AMP + PPi (by aminoacyl synthetase) AA~AMP + tRNA Æ AA~tRNA + AMP AA~tRNA + growing polypeptide (n) Æ tRNA + growing polypeptide (n+1) Nucleic acid precursors are activated by the presence of PPi -DNA and RNA are built up from mononucleotide (nucleoside phosphate) -phosphodiester bond releases considerable free energy upon hydrolysis (-6 kcal/mole) Æ mean that nucleic acids will spontaneously hydrolyze Æ need even highly activated precursors for the synthesis -Precursors for DNA: dATP, dGTP, dCTP, dTTP -Precursors for RNA: ATP, GTP, CTP, UTP Deoxynucleoside-P + ATP Æ deoxynucleoside-P~P (i.e., dNDP) + ADP Deoxynucleoside-P~P + ATP Æ deoxynucleoside-P~P~P (i.e., dNTP) + ADP deoxynucleoside-P~P~P + polynucleotide (n) Æ P~P + polynucleotide (n+1) -ΔG of the reaction ~ 0.5 kcal/mole Æ what is the source of free energy The value of P~P release in nucleic acid synthesis -needed free energy comes from the splitting (분열) of PPi group P~P Æ 2P (ΔG = -7 kcal/mole) -reactions with small, positive ΔG value are often part of important metabolic pathway in which they are followed by reactions with large negative ΔG value -single reaction never occurs independently, rather the nature of the equilibrium is constantly being changed through the addition and removal of metabolites P~P splits characterize most biosynthetic reactions -all biosynthetic reactions are characterized by one or more steps that release P~P group (e.g., synthesis of polypeptide, polynucleotide) -compare dNTP and dNDP as precursors for DNA synthesis (Fig 5) -according to the law of mass action, “a” reaction would become reversible -split of P~P provide the driving force to forward reaction -Thus, (d)NTP, instead of (d)NDP, is not a matter of chance Figure 5