In silico aided metaoblic engineering of Saccharomyces
... can be redirected towards ethanol by increasing the consumption of ATP for biomass production or reducing the amount of ATP formed in association with ethanol production. (Nissen et al. 2000) • Deletion of the structural genes in glycerol biosynthesis is not a successful strategy. • The maximum spec ...
... can be redirected towards ethanol by increasing the consumption of ATP for biomass production or reducing the amount of ATP formed in association with ethanol production. (Nissen et al. 2000) • Deletion of the structural genes in glycerol biosynthesis is not a successful strategy. • The maximum spec ...
ATP - LSU School of Medicine
... • Protons transported from the matrix to the inner mitochondrial space results in an electric gradient and a pH gradient • As the protons flow through the membrane channel back into the matrix they drive ATP synthesis Occurs with energy utilized by ATP synthase This proton transport couples electron ...
... • Protons transported from the matrix to the inner mitochondrial space results in an electric gradient and a pH gradient • As the protons flow through the membrane channel back into the matrix they drive ATP synthesis Occurs with energy utilized by ATP synthase This proton transport couples electron ...
Review for Final Summer 2011
... o Fermentation Glycolysis splits sugar to make ATP & NADH Pyruvate from Glycolysis either enter the mitochondria (cellular respiration) or stays in cytosol (one of the two types of fermentation) Fermentation: Alcohol vs. lactic acid Why would a cell do fermentation instead of cellular respir ...
... o Fermentation Glycolysis splits sugar to make ATP & NADH Pyruvate from Glycolysis either enter the mitochondria (cellular respiration) or stays in cytosol (one of the two types of fermentation) Fermentation: Alcohol vs. lactic acid Why would a cell do fermentation instead of cellular respir ...
Dominant Dietary Fatty Acids
... o Ying-yang…fully activated at both ends of enzymes o Why would mammals benefit from 7 sites? Tends to be a little more efficient The players are all together, substrate there Fig 14.2 o The 3 steps missing from the cycle The reductive steps in fatty acid synthesis o The reductant is NADPH rathe ...
... o Ying-yang…fully activated at both ends of enzymes o Why would mammals benefit from 7 sites? Tends to be a little more efficient The players are all together, substrate there Fig 14.2 o The 3 steps missing from the cycle The reductive steps in fatty acid synthesis o The reductant is NADPH rathe ...
Energy Continuum/The Recovery Process (b) Explain the term
... 6 this system can only partially breakdown carbohydrate and cannot break down fat 7 there are only limited stores of carbohydrate 8 only produces limited amount of ATP / 2 ATP Aerobic system 9 this system does not produce fatiguing by-products / carbon dioxide and water 10 this system can also break ...
... 6 this system can only partially breakdown carbohydrate and cannot break down fat 7 there are only limited stores of carbohydrate 8 only produces limited amount of ATP / 2 ATP Aerobic system 9 this system does not produce fatiguing by-products / carbon dioxide and water 10 this system can also break ...
File
... Disaccharides- “double sugars” Formed when two simple sugars are joined by dehydration synthesis. In this reaction a water molecule is lost as the bond is formed. ...
... Disaccharides- “double sugars” Formed when two simple sugars are joined by dehydration synthesis. In this reaction a water molecule is lost as the bond is formed. ...
Muscle Physiology - Brookville Local Schools
... myosin to break down ATP more rapidly, less blood supply, fewer and smaller mitochondria than slowtwitch ...
... myosin to break down ATP more rapidly, less blood supply, fewer and smaller mitochondria than slowtwitch ...
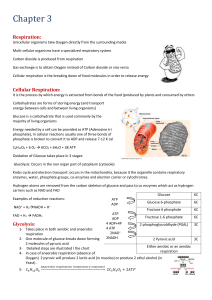
4.4 Overview of Cellular Respiration
... 4.4 Overview of Cellular Respiration Glycolysis is needed for cellular respiration. • The products of glycolysis enter cellular respiration when oxygen is available. – two ATP molecules are used to split glucose – four ATP molecules are produced – two molecules of NADH produced – two molecules of p ...
... 4.4 Overview of Cellular Respiration Glycolysis is needed for cellular respiration. • The products of glycolysis enter cellular respiration when oxygen is available. – two ATP molecules are used to split glucose – four ATP molecules are produced – two molecules of NADH produced – two molecules of p ...
Overview of Metabolism - Chapter 4 - Formatted
... of dynamic equilibrium, constantly taking in substances from its external environment, processing them, synthesizing its own requirements, degrading what is old or harmful, and sending out what it may have produced for other cells, or what is waste. Since all these processes either require energy or ...
... of dynamic equilibrium, constantly taking in substances from its external environment, processing them, synthesizing its own requirements, degrading what is old or harmful, and sending out what it may have produced for other cells, or what is waste. Since all these processes either require energy or ...
reactions --- electrons can`t flow in a vacuum, oxidation reactions
... 1.) Break the redox system down into multiple smaller steps, each of which release a manageable amount of energy 2.) Use mobile electron carriers to link these smaller reactions ...
... 1.) Break the redox system down into multiple smaller steps, each of which release a manageable amount of energy 2.) Use mobile electron carriers to link these smaller reactions ...
BIOL 1406 Discussion Questions: Photosynthesis
... photosynthetic pigments to be found in species that live in these different habitats? Why or why not? How would you test your hypothesis? ...
... photosynthetic pigments to be found in species that live in these different habitats? Why or why not? How would you test your hypothesis? ...
Complete the following
... Yeast respiration produces carbon dioxide Another Kind of fermentation is acidic fermentation: 1- Carried out by several kinds of bacteria. 2- It produces acid instead of alcohol 3- Many milk products (Cheese, butter and yoghurt) are manufactured by this kind of fermentation. Seeds of angiosperms ca ...
... Yeast respiration produces carbon dioxide Another Kind of fermentation is acidic fermentation: 1- Carried out by several kinds of bacteria. 2- It produces acid instead of alcohol 3- Many milk products (Cheese, butter and yoghurt) are manufactured by this kind of fermentation. Seeds of angiosperms ca ...
High Energy compounds
... • 2,3BPG is used as a mechanism to oversee the efficient release of oxygen from hemoglobin. • Low oxygen levels trigger a rise in 1,3BPG levels which in turn raises the level of 2,3BPG which alters the efficiency of oxygen dissociation from hemoglobin. ...
... • 2,3BPG is used as a mechanism to oversee the efficient release of oxygen from hemoglobin. • Low oxygen levels trigger a rise in 1,3BPG levels which in turn raises the level of 2,3BPG which alters the efficiency of oxygen dissociation from hemoglobin. ...
Unit 3 Biochemistry - The Naked Science Society
... usually associated with living things. always contain CARBON. are “large” molecules, with many atoms always have covalent bonds (share electrons) ...
... usually associated with living things. always contain CARBON. are “large” molecules, with many atoms always have covalent bonds (share electrons) ...
Respiration and Photosynthesis Class Work Where does the energy
... respiration energy is released through the breakdown of glucose and used to create ATP. ATP is an energy-storing molecule that can be broken down to ADP to release energy to drive other cellular processes. Both photosynthesis and cellular respiration convert energy from one form to another, but neit ...
... respiration energy is released through the breakdown of glucose and used to create ATP. ATP is an energy-storing molecule that can be broken down to ADP to release energy to drive other cellular processes. Both photosynthesis and cellular respiration convert energy from one form to another, but neit ...
Exam #2 Review
... pathways must be dynamic and coordinated so that cells can respond to changes in environment. Each reaction is catalyzed by a specific enzyme. Every enzyme-catalyzed reaction represents a potential point of regulation (inhibition or activation). In catabolic pathways the starting compound (an ...
... pathways must be dynamic and coordinated so that cells can respond to changes in environment. Each reaction is catalyzed by a specific enzyme. Every enzyme-catalyzed reaction represents a potential point of regulation (inhibition or activation). In catabolic pathways the starting compound (an ...
Catalogue Number CTK-468 Introduction Insulin decreases blood
... contains an intrachain disulfide bond. Insulin regulates the cellular uptake, utilization, and storage of glucose, amino acids, and fatty acids and inhibits the breakdown of glycogen, protein, and fat. Insulin Porcine is purified by proprietary chromatographic techniques. ...
... contains an intrachain disulfide bond. Insulin regulates the cellular uptake, utilization, and storage of glucose, amino acids, and fatty acids and inhibits the breakdown of glycogen, protein, and fat. Insulin Porcine is purified by proprietary chromatographic techniques. ...
Ch 9 Notes - Dublin City Schools
... membrane couples the redox reactions of the electron transport chain to ATP synthesis • The H+ gradient is referred to as a protonmotive force, emphasizing its capacity to do ...
... membrane couples the redox reactions of the electron transport chain to ATP synthesis • The H+ gradient is referred to as a protonmotive force, emphasizing its capacity to do ...
1. Amino Acids,Peptides, Proteins
... 16. The Pentose Phosphate Pathway and Other Pathways of Hexose Metabolism Ch. 20. The Pentose Phosphate Pathway & Other Pathways of Hexose Metabolism - without the metabolism of aminosugars 17. Overview of Glucose Metabolism. Control of the Blood Glucose Ch. 19. Gluconeogenesis & the Control of Bloo ...
... 16. The Pentose Phosphate Pathway and Other Pathways of Hexose Metabolism Ch. 20. The Pentose Phosphate Pathway & Other Pathways of Hexose Metabolism - without the metabolism of aminosugars 17. Overview of Glucose Metabolism. Control of the Blood Glucose Ch. 19. Gluconeogenesis & the Control of Bloo ...
Oxidation
... • Can be used as a fuel in most tissues and organs. • Formation occurs when the amount of acetyl CoA produced is excessive compared to the amount of oxaloacetate available to react with it and take it into the ...
... • Can be used as a fuel in most tissues and organs. • Formation occurs when the amount of acetyl CoA produced is excessive compared to the amount of oxaloacetate available to react with it and take it into the ...
Cellular Respiration - Hss-1.us
... ATP energy beyond that obtained form glycolysis. – Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose, C6H12O6, into pyruvate, C3H3O3-. The free energy released in this process is used to form the high energy compounds, ATP (adeno ...
... ATP energy beyond that obtained form glycolysis. – Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose, C6H12O6, into pyruvate, C3H3O3-. The free energy released in this process is used to form the high energy compounds, ATP (adeno ...
Guide 1406 Ch, 1-5
... What is the difference between polymers and monomers? The blood cholesterol levels and where can you find most cholesterol? What are amino acids? What type of linkages join glucose, amino acids, fatty acids and nucleotides together? What are the levels of protein structure? What is meant for protein ...
... What is the difference between polymers and monomers? The blood cholesterol levels and where can you find most cholesterol? What are amino acids? What type of linkages join glucose, amino acids, fatty acids and nucleotides together? What are the levels of protein structure? What is meant for protein ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑