11/8/09 Chapter 3 Biochemistry Section 1 Carbon Compounds
... characteristics of the molecules they compose and the chemical reactions the molecules undergo. For example, one functional group to living things, the hydroxyl group, -OH, can make the molecule it is attached to polar. Polar molecules are hydrophilic, or soluble in water. An alcohol is an organic c ...
... characteristics of the molecules they compose and the chemical reactions the molecules undergo. For example, one functional group to living things, the hydroxyl group, -OH, can make the molecule it is attached to polar. Polar molecules are hydrophilic, or soluble in water. An alcohol is an organic c ...
Exercise Metabolism
... remains relatively stable. • Plateau of oxygen uptake is known as steady rate. ...
... remains relatively stable. • Plateau of oxygen uptake is known as steady rate. ...
Uncommon pathways of metabolism among lactic acid bacteria
... NAD+-dependent protein mediates the entire reaction. Because free NADH is not produced by the Lb. plantarum enzyme, the reaction appears to be fundamentally decarboxylative in nature [25,28]. Immunological studies with anti-Lb. plantarum malie enzyme suggests that the antigenic structure of the prot ...
... NAD+-dependent protein mediates the entire reaction. Because free NADH is not produced by the Lb. plantarum enzyme, the reaction appears to be fundamentally decarboxylative in nature [25,28]. Immunological studies with anti-Lb. plantarum malie enzyme suggests that the antigenic structure of the prot ...
File
... Pasteur observed that yeast consumes far more glucose when growing under anaerobic conditions than when growing under aerobic conditions. Scientists now know that the rate of ATP production by anaerobic glycolysis can be up to 100 times faster than that of oxidative phosphorylation, but much glucose ...
... Pasteur observed that yeast consumes far more glucose when growing under anaerobic conditions than when growing under aerobic conditions. Scientists now know that the rate of ATP production by anaerobic glycolysis can be up to 100 times faster than that of oxidative phosphorylation, but much glucose ...
FinalReview
... (a) Energy is released when electrons are moved from an energy source with a low affinity for electrons to a terminal electron acceptor with a higher affinity. ...
... (a) Energy is released when electrons are moved from an energy source with a low affinity for electrons to a terminal electron acceptor with a higher affinity. ...
Units of Competency
... broken down by a very complicated series of reactions. In humans, the end product is lactic acid; ATP is also formed. Lactic acid is actually a by‐product, and when it accumulates to high levels, it causes muscular fatigue. The production of ATP, on the other hand, is the sole purpose of glyc ...
... broken down by a very complicated series of reactions. In humans, the end product is lactic acid; ATP is also formed. Lactic acid is actually a by‐product, and when it accumulates to high levels, it causes muscular fatigue. The production of ATP, on the other hand, is the sole purpose of glyc ...
Metabolism: Dissimilatory (energy, catabolic) metabolism
... Autotrophy: solely dependent on inorganic compounds Heterotrophy: dependent on organic substrates Distinction rarely absolute: ”autrotrophs” may require organic growth factors (vitamins). Usually refers to carbon source for assimilatory metabolism (autotrophs depend on C-1 compounds, that is CO2 or ...
... Autotrophy: solely dependent on inorganic compounds Heterotrophy: dependent on organic substrates Distinction rarely absolute: ”autrotrophs” may require organic growth factors (vitamins). Usually refers to carbon source for assimilatory metabolism (autotrophs depend on C-1 compounds, that is CO2 or ...
ENZYME STRUCTURE AND FUNCTION
... very difficult problem that has not yet been solved. Most enzymes are much larger than the substrates they act on, and only a small portion of the enzyme (around 2–4 amino acids) is directly involved in catalysis. The region that contains these catalytic residues, binds the substrate, and then carri ...
... very difficult problem that has not yet been solved. Most enzymes are much larger than the substrates they act on, and only a small portion of the enzyme (around 2–4 amino acids) is directly involved in catalysis. The region that contains these catalytic residues, binds the substrate, and then carri ...
Introduction to Biology
... They contain Hydrogen, carbon and oxygen in their structure in addition to phosphorus and sulphur as phospholipids that present in the cell membrane of animal and plants. Phospholipids are similar to fat molecules in the structure except for the phosphate group (P O4) which replaced the third fatty ...
... They contain Hydrogen, carbon and oxygen in their structure in addition to phosphorus and sulphur as phospholipids that present in the cell membrane of animal and plants. Phospholipids are similar to fat molecules in the structure except for the phosphate group (P O4) which replaced the third fatty ...
Chapter 6
... • However, these fuels do not spontaneously combine with O2 because they lack the activation energy. • That’s where enzymes come in. • Enzymes lower the barrier of activation energy of suitable food molecules, allowing these “nutritional fuels” to be oxidized slowly. ...
... • However, these fuels do not spontaneously combine with O2 because they lack the activation energy. • That’s where enzymes come in. • Enzymes lower the barrier of activation energy of suitable food molecules, allowing these “nutritional fuels” to be oxidized slowly. ...
MACROMOLECULES - Savitha Sastry
... How do polymers break up? Hydrolysis Reaction: Covalent Bond is broken; H2O is added across the broken bond Polymers make Monomers Provides ATP and Uses Enzymes Used for digestion, cell respiration ...
... How do polymers break up? Hydrolysis Reaction: Covalent Bond is broken; H2O is added across the broken bond Polymers make Monomers Provides ATP and Uses Enzymes Used for digestion, cell respiration ...
ExamReview2012
... 32. Enzyme inhibition (competitive and non-competitive) and allosteric regulation 33. Cofactors and coenzymes ...
... 32. Enzyme inhibition (competitive and non-competitive) and allosteric regulation 33. Cofactors and coenzymes ...
chapter 2 the origin and chemistry of life
... 3. Glucose is commonly found in the blood of animals and is an important immediate energy source for cells. (Figure 2.6, 2.7) 4. Cellulose occurs in greater quantities than all other organic materials combined. 5. Carbohydrates, synthesized by plants by photosynthesis, are the starting point of food ...
... 3. Glucose is commonly found in the blood of animals and is an important immediate energy source for cells. (Figure 2.6, 2.7) 4. Cellulose occurs in greater quantities than all other organic materials combined. 5. Carbohydrates, synthesized by plants by photosynthesis, are the starting point of food ...
integrated-principles-of-zoology-16th-edition-hickman
... 3. Glucose is commonly found in the blood of animals and is an important immediate energy source for cells. (Figure 2.6, 2.7) 4. Cellulose occurs in greater quantities than all other organic materials combined. 5. Carbohydrates, synthesized by plants by photosynthesis, are the starting point of food ...
... 3. Glucose is commonly found in the blood of animals and is an important immediate energy source for cells. (Figure 2.6, 2.7) 4. Cellulose occurs in greater quantities than all other organic materials combined. 5. Carbohydrates, synthesized by plants by photosynthesis, are the starting point of food ...
The Major Transitions in Evolution
... • Look for those molecules that yield the largest increase in metabolic scope • Stop when there is a functional metabolism • Check the results with flux balance analysis (FBA) for the producible compounds in steady state ...
... • Look for those molecules that yield the largest increase in metabolic scope • Stop when there is a functional metabolism • Check the results with flux balance analysis (FBA) for the producible compounds in steady state ...
Bacterial Classification
... – Metabolism - sum of all chemical reactions in cell – Anabolism - reactions that synthesize or “build up” e.g. protein synthesis – Catabolism - reactions that digest or “break down” e.g. starch to glucose ...
... – Metabolism - sum of all chemical reactions in cell – Anabolism - reactions that synthesize or “build up” e.g. protein synthesis – Catabolism - reactions that digest or “break down” e.g. starch to glucose ...
A1985AFW3400002
... Surprisingly, all three have been 12 featured in the Citation Classics series. The present paper together 2 with the one on insulin radio. immunoassay represents the work I carried out for my PhD as a graduate student working under the supervision of Philip Randle. I was very fortunate to be a membe ...
... Surprisingly, all three have been 12 featured in the Citation Classics series. The present paper together 2 with the one on insulin radio. immunoassay represents the work I carried out for my PhD as a graduate student working under the supervision of Philip Randle. I was very fortunate to be a membe ...
Caffeic acid in lowering blood glucose in the application
... postprandial blood glucose has good inhibition in the experimental group lower postprandial blood glucose than the control group 23% -55%. Caffeic acid in diabetic rat renal medulla by stimulating the secretion of B endorphin-like substance into the sky hypoglycemic effect, as in normal mice was not ...
... postprandial blood glucose has good inhibition in the experimental group lower postprandial blood glucose than the control group 23% -55%. Caffeic acid in diabetic rat renal medulla by stimulating the secretion of B endorphin-like substance into the sky hypoglycemic effect, as in normal mice was not ...
Some prokaryotes use anaerobic respiration in which
... Anaerobic respiration is a type of respiration where oxygen is not used; instead, organic or inorganic molecules are used as final electron acceptors. Fermentation includes processes that use an organic molecule to regenerate NAD+ from NADH. Types of fermentation include lactic acid fermentation and ...
... Anaerobic respiration is a type of respiration where oxygen is not used; instead, organic or inorganic molecules are used as final electron acceptors. Fermentation includes processes that use an organic molecule to regenerate NAD+ from NADH. Types of fermentation include lactic acid fermentation and ...
1 APPENDIX 1 TEST PRINCIPLES USED IN THE BIOCHEMICAL
... Triglycerides are hydrolysed by lipoprotein lipase to glycerol followed by oxidation to dihydroxyacetone phosphate and hydrogen peroxide. The hydrogen oxide produced then reacts with 4-aminophenazone and 4-4-chlorophenol under the catalytic action of peroxidase to form a red dyestuff (Trinder end po ...
... Triglycerides are hydrolysed by lipoprotein lipase to glycerol followed by oxidation to dihydroxyacetone phosphate and hydrogen peroxide. The hydrogen oxide produced then reacts with 4-aminophenazone and 4-4-chlorophenol under the catalytic action of peroxidase to form a red dyestuff (Trinder end po ...
The Cardiovascular System and Exercise
... During resting conditions the oxygen content of blood varies from about 20ml of oxygen per 100ml of arterial blood to 14ml of oxygen per 100ml of venous blood. The difference in oxygen content of arterial and venous blood is known as a-vO2 difference. As exercise intensity increase the a-vO2 differe ...
... During resting conditions the oxygen content of blood varies from about 20ml of oxygen per 100ml of arterial blood to 14ml of oxygen per 100ml of venous blood. The difference in oxygen content of arterial and venous blood is known as a-vO2 difference. As exercise intensity increase the a-vO2 differe ...
Photsynthesis III - Light Indpendent
... – 3 Carbon Dioxides enter the cycle and combine with RuBP to create 3 very unstable 6 carbon molecules. – These 6-carbon molecules instantly break down into 6, 3-carbon molecules that are much more stable. – We now have all of the carbons in the cycle that are needed to make ½ of a glucose molecule. ...
... – 3 Carbon Dioxides enter the cycle and combine with RuBP to create 3 very unstable 6 carbon molecules. – These 6-carbon molecules instantly break down into 6, 3-carbon molecules that are much more stable. – We now have all of the carbons in the cycle that are needed to make ½ of a glucose molecule. ...
3 hours - The University of Winnipeg
... Question 4 (4 points): A cell contains inhibitors for pyruvate kinase (PEP ? pyruvate) and glyceraldehyde-3-phosphate dehydrogenase (glyceraldehyde-3-P ? 1,3-bisphosphoglycerate). These inhibitors completely inhibit these enzymes. The cell is fed 2-phosphoglycerate enriched with 14C in carbon-1. Th ...
... Question 4 (4 points): A cell contains inhibitors for pyruvate kinase (PEP ? pyruvate) and glyceraldehyde-3-phosphate dehydrogenase (glyceraldehyde-3-P ? 1,3-bisphosphoglycerate). These inhibitors completely inhibit these enzymes. The cell is fed 2-phosphoglycerate enriched with 14C in carbon-1. Th ...
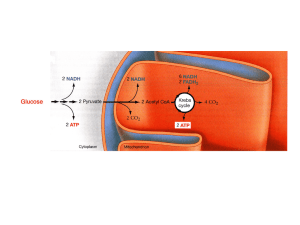
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑