Chapter 10:Alcohols, Phenols and Ethers
... The ethers have boiling points that are closer to those of alkanes with similar molecular weights. compares the dipole moments of dimethyl ether, diethyl ether, and tetrahydrofuran (THF) with those of alkanes and alcohols of similar molecular weights. An ether such as THF provides a strongly polar s ...
... The ethers have boiling points that are closer to those of alkanes with similar molecular weights. compares the dipole moments of dimethyl ether, diethyl ether, and tetrahydrofuran (THF) with those of alkanes and alcohols of similar molecular weights. An ether such as THF provides a strongly polar s ...
Chemistry
... JOHN MCMURRY, educated at Harvard and Columbia, has taught approximately 17,000 students in general and organic chemistry over a 30-year period. A Professor of Chemistry at Cornell University since 1980, Dr. McMurry previously spent 13 years on the faculty at the University of California at Santa Cr ...
... JOHN MCMURRY, educated at Harvard and Columbia, has taught approximately 17,000 students in general and organic chemistry over a 30-year period. A Professor of Chemistry at Cornell University since 1980, Dr. McMurry previously spent 13 years on the faculty at the University of California at Santa Cr ...
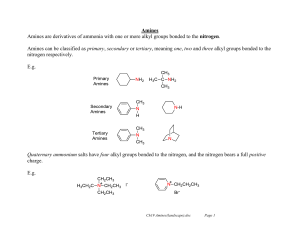
Amines Amines are derivatives of ammonia with one or more alkyl
... The use of the lone pair to form a bond to hydrogen (i.e. protonation) removes the lone pair from the system, and this makes the protonated form no longer aromatic - this is energetically unfavorable. ...
... The use of the lone pair to form a bond to hydrogen (i.e. protonation) removes the lone pair from the system, and this makes the protonated form no longer aromatic - this is energetically unfavorable. ...
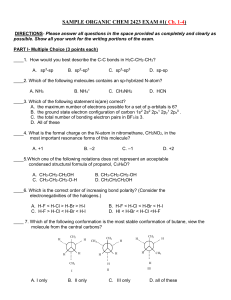
organic practice problems
... ____ 60. Which of the following molecules is ethanol? a. C2H6 b. CH3CO2H c. CH3CHO d. CH3CH2OH e. CH3OCH3 ____ 61. Which functional group does not contain an oxygen atom? a. alcohol b. amine c. amide d. ester e. ether ____ 62. The functional group RCO2R' is characteristic of an ________. a. ether b. ...
... ____ 60. Which of the following molecules is ethanol? a. C2H6 b. CH3CO2H c. CH3CHO d. CH3CH2OH e. CH3OCH3 ____ 61. Which functional group does not contain an oxygen atom? a. alcohol b. amine c. amide d. ester e. ether ____ 62. The functional group RCO2R' is characteristic of an ________. a. ether b. ...
Syllabus and Regulations for 2-year, 4
... Questions will be set by internal teachers and guest faculties, which will be ...
... Questions will be set by internal teachers and guest faculties, which will be ...
Kinetic Modeling Of Methanol Synthesis From Carbon Monoxide
... (∆H = -41.2 kJ/mol; ∆G = -28.60 kJ/mol) ...
... (∆H = -41.2 kJ/mol; ∆G = -28.60 kJ/mol) ...
hydrogen peroxide disproportionation and organic
... Reactivity of electrophilic olefins with nucleophilic oxidants, such as hydroperoxide, react to produce the epoxide plus the oxidants’ corresponding leaving group, in this case hydroxide.........................................................................7 ...
... Reactivity of electrophilic olefins with nucleophilic oxidants, such as hydroperoxide, react to produce the epoxide plus the oxidants’ corresponding leaving group, in this case hydroxide.........................................................................7 ...
Chem. Soc. Rev., 2015, 44, 2202--2220 - RSC Publishing
... accommodates both the boron atom and the weakly Lewis acidic silicon atom. The hydride is directly transferred from the silicon atom to the highly electrophilic carbonyl carbon atom. The fundamentally different activation modes exerted by BF3 (CQO group) and B(C6F5)3 (Si–H bond) were mainly attribut ...
... accommodates both the boron atom and the weakly Lewis acidic silicon atom. The hydride is directly transferred from the silicon atom to the highly electrophilic carbonyl carbon atom. The fundamentally different activation modes exerted by BF3 (CQO group) and B(C6F5)3 (Si–H bond) were mainly attribut ...
NAME - HCC Learning Web
... The conjugate acid to (CH3)3N: is (CH3)3NH+. An electron pair donor substance considers being a Lewis acid. The stronger the acid, the weaker the conjugate base. ...
... The conjugate acid to (CH3)3N: is (CH3)3NH+. An electron pair donor substance considers being a Lewis acid. The stronger the acid, the weaker the conjugate base. ...
c8h18 isomers
... ♦ The molecules are non-polar or very weakly polar ♦ The forces holding together non-polar molecules are van der Waals forces. These intermolecular forces, which operate only over very small distances, result from induced polarization of the electron clouds in molecules. ♦ Within a family: The large ...
... ♦ The molecules are non-polar or very weakly polar ♦ The forces holding together non-polar molecules are van der Waals forces. These intermolecular forces, which operate only over very small distances, result from induced polarization of the electron clouds in molecules. ♦ Within a family: The large ...
Palladium(II)-Catalyzed Oxidative Cyclization Strategies Andreas K. Å. Persson
... palladium(II) readily forms π-complexes with a wide range of unsaturated hydrocarbons such as alkynes, alkenes and allenes. The coordination to palladium renders these fragments susceptible towards nucleophilic attack and/or migratory insertion. Many palladium(II)-catalyzed oxidative processes, suc ...
... palladium(II) readily forms π-complexes with a wide range of unsaturated hydrocarbons such as alkynes, alkenes and allenes. The coordination to palladium renders these fragments susceptible towards nucleophilic attack and/or migratory insertion. Many palladium(II)-catalyzed oxidative processes, suc ...
Designing Organic Synthesis - Department of Chemistry, IIT Bombay
... Theses simple targets were synthesized by often starting with compounds which are closely related to products These became impractical when the targets became more complex To tackle this, higher level of intellectual planning and skill are required Better understanding of reaction mechanisms A worki ...
... Theses simple targets were synthesized by often starting with compounds which are closely related to products These became impractical when the targets became more complex To tackle this, higher level of intellectual planning and skill are required Better understanding of reaction mechanisms A worki ...
Transition Metal Reagents and Catalysts
... ®rst by giving a simple mechanistic explanation in chapter 2. Then a number of important types of reactions classi®ed mainly by representative substrates such as organic halides and allylic derivatives are surveyed with pertinent examples. For this purpose, I cited many references; these were select ...
... ®rst by giving a simple mechanistic explanation in chapter 2. Then a number of important types of reactions classi®ed mainly by representative substrates such as organic halides and allylic derivatives are surveyed with pertinent examples. For this purpose, I cited many references; these were select ...
Functional Groups
... • Ethanol: This molecule has an OH group attached to its backbone. It is called a hydroxy functional group. Ethanol has lone pairs and polar bonds that make it reactive with a variety of reagents. The hydroxy group makes the properties of ethanol very different from the properties of ethane. ...
... • Ethanol: This molecule has an OH group attached to its backbone. It is called a hydroxy functional group. Ethanol has lone pairs and polar bonds that make it reactive with a variety of reagents. The hydroxy group makes the properties of ethanol very different from the properties of ethane. ...
102MSJc14 - Louisiana Tech University
... completion, that is, until one of the reactants runs out. Many reactions do proceed essentially to completion: complete reactions are indicated by . For such reactions it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...
... completion, that is, until one of the reactants runs out. Many reactions do proceed essentially to completion: complete reactions are indicated by . For such reactions it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...
iNTRODUCTiON TO ORGANiC COMPOUNDS
... The last group of compounds we are going to discuss includes some that are of great importance and interest today. Included are the chlorofluorocarbons that are used in refrigeration and air conditioning systems and which are thought to be involved in the depletion of ozone in the upper atmosphere. ...
... The last group of compounds we are going to discuss includes some that are of great importance and interest today. Included are the chlorofluorocarbons that are used in refrigeration and air conditioning systems and which are thought to be involved in the depletion of ozone in the upper atmosphere. ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.