Investigation of the steric and electronic properties of 3
... formation. Diversification of the hydroamination reaction’s substrate scope will provide key tools for organic chemists to make C-N bond containing products with greater ease and variety, allowing for the use of less reagents, time, and energy, therefore effecting a cost savings in these processes. ...
... formation. Diversification of the hydroamination reaction’s substrate scope will provide key tools for organic chemists to make C-N bond containing products with greater ease and variety, allowing for the use of less reagents, time, and energy, therefore effecting a cost savings in these processes. ...
an introduction to organic reactions
... saying that Organic Chemistry really isn't that bad and can, in fact, be pretty interesting. I think it's important to understand from the start that this is completely true…I can almost assure you that you will enjoy Orgo much more than General Chemistry, and the work & endash; although there may b ...
... saying that Organic Chemistry really isn't that bad and can, in fact, be pretty interesting. I think it's important to understand from the start that this is completely true…I can almost assure you that you will enjoy Orgo much more than General Chemistry, and the work & endash; although there may b ...
The chemistry of beer aging – a critical review Food Chemistry
... generalization of the sensory evolution during beer storage and is by no means applicable to every beer. A constant decrease in bitterness is observed during aging. This is partly due to sensory masking by an increasing sweet taste. In contrast to an initial acceleration of sweet aroma development, ...
... generalization of the sensory evolution during beer storage and is by no means applicable to every beer. A constant decrease in bitterness is observed during aging. This is partly due to sensory masking by an increasing sweet taste. In contrast to an initial acceleration of sweet aroma development, ...
Alcohols - Angelo State University
... • Common names for ethers are obtained by first naming the two carbon groups attached to the oxygen (in alphabetical order) and then adding the word “ether” to the end. If the two groups are the same, the prefix “di-” is used, although sometimes this is simply dropped (“ethyl ether”). ...
... • Common names for ethers are obtained by first naming the two carbon groups attached to the oxygen (in alphabetical order) and then adding the word “ether” to the end. If the two groups are the same, the prefix “di-” is used, although sometimes this is simply dropped (“ethyl ether”). ...
irm_ch15
... 15.29 Common names for ketones are similar to the common names used for ethers with the carbonyl group taking the place of the oxygen atom. The alkyl groups are named, and the word ketone is added as a separate word: alkyl alkyl ketone or dialkyl ketone. Three ketones have additional common names; a ...
... 15.29 Common names for ketones are similar to the common names used for ethers with the carbonyl group taking the place of the oxygen atom. The alkyl groups are named, and the word ketone is added as a separate word: alkyl alkyl ketone or dialkyl ketone. Three ketones have additional common names; a ...
Iodine and Lipase Based Green Oxidation Technology
... Generally speaking, formation of waste during any process should be judged according to the E factor. 29, 30 Solvents that are less toxic, less harmful and that can be easily recovered and recycled are the desired ones. 1.1.4 Recovery and recycling Ease of recovery of reagents an ...
... Generally speaking, formation of waste during any process should be judged according to the E factor. 29, 30 Solvents that are less toxic, less harmful and that can be easily recovered and recycled are the desired ones. 1.1.4 Recovery and recycling Ease of recovery of reagents an ...
Multiple Choice Exam Review June 2016
... ____ 35. The value of the rate constant, k, is valid only for a specific reaction at a specific temperature. _________________________ ____ 36. An ineffective collision is one that has sufficient energy and correct orientation so that the reaction can proceed. _________________________ ____ 37. The ...
... ____ 35. The value of the rate constant, k, is valid only for a specific reaction at a specific temperature. _________________________ ____ 36. An ineffective collision is one that has sufficient energy and correct orientation so that the reaction can proceed. _________________________ ____ 37. The ...
Course Notes
... Nuclei of bonded atoms undergo vibrations similar to two balls connected by a spring. Depending on the particular atoms bonded to each other (and their masses) the frequency of this vibration will vary. Infrared energy is absorbed by molecules resulting in an excited vibrational state. Vibrations oc ...
... Nuclei of bonded atoms undergo vibrations similar to two balls connected by a spring. Depending on the particular atoms bonded to each other (and their masses) the frequency of this vibration will vary. Infrared energy is absorbed by molecules resulting in an excited vibrational state. Vibrations oc ...
Lithium Iodide Original Commentary - Groupe Charette
... LiI as an Additive for Organometallic-mediated Transformations. Diastereoselectivity in the cyclization of 5-hexenyllithiums was shown to be influenced by LiI.34 As an additive in the reduction of α,β-unsaturated ketones by Bu2 SnH2 /Bu2 SnF2 , LiI has a dramatic effect on the selectivity for 1,2- ve ...
... LiI as an Additive for Organometallic-mediated Transformations. Diastereoselectivity in the cyclization of 5-hexenyllithiums was shown to be influenced by LiI.34 As an additive in the reduction of α,β-unsaturated ketones by Bu2 SnH2 /Bu2 SnF2 , LiI has a dramatic effect on the selectivity for 1,2- ve ...
N - Clayton State University
... Methanol, CH3OH, called methyl alcohol, is a common solvent, a fuel additive, produced in large quantities Ethanol, CH3CH2OH, called ethyl alcohol, is a solvent, fuel, beverage OH groups bonded to vinylic sp2-hybridized carbons are called enols ...
... Methanol, CH3OH, called methyl alcohol, is a common solvent, a fuel additive, produced in large quantities Ethanol, CH3CH2OH, called ethyl alcohol, is a solvent, fuel, beverage OH groups bonded to vinylic sp2-hybridized carbons are called enols ...
chemistry - Textbooks Online
... (i) The magnitude of order of a reaction may be zero, or fractional or integral values. For an elementary reaction, its order is never fractional since it is a one step process. (ii) Order of a reaction should be determined only by experiments. It cannot be predicted interms of stoichiometry of reac ...
... (i) The magnitude of order of a reaction may be zero, or fractional or integral values. For an elementary reaction, its order is never fractional since it is a one step process. (ii) Order of a reaction should be determined only by experiments. It cannot be predicted interms of stoichiometry of reac ...
The Organometallic Chemistry Of Transition Metals
... Chelate Effect Other ligands can have more than one donor atom, each with its lone pair; an example is ethylenediamine (NH2 CH2 CH2 NH2 , often abbreviated “en”). Such ligands most commonly donate both lone pairs to the same metal to give a ring compound, known as a chelate, from the Greek word for ...
... Chelate Effect Other ligands can have more than one donor atom, each with its lone pair; an example is ethylenediamine (NH2 CH2 CH2 NH2 , often abbreviated “en”). Such ligands most commonly donate both lone pairs to the same metal to give a ring compound, known as a chelate, from the Greek word for ...
Unit 2
... CH3 The main carbon chain is ________________, so you may be tempted to call this molecule __________________. This is, however, not necessary because there is no other place for the methyl group to exist on the main chain without the molecule becoming butane. This particular molecule may be ...
... CH3 The main carbon chain is ________________, so you may be tempted to call this molecule __________________. This is, however, not necessary because there is no other place for the methyl group to exist on the main chain without the molecule becoming butane. This particular molecule may be ...
Document
... Classes of Organic Compounds (cont’d) 4. Carboxylic Acids: are organic compounds that contain the carboxyl functional group. A member of this class of organic compounds can be represented by the general formula: ...
... Classes of Organic Compounds (cont’d) 4. Carboxylic Acids: are organic compounds that contain the carboxyl functional group. A member of this class of organic compounds can be represented by the general formula: ...
ANNEX (Manuscrits posteriors a la Comissió de Doctorat de Juliol del...
... The synthesis of [3]--[6]- has shown that the modified Kumada reaction can be applied to produce B-C bonds for di-substituted cobaltabisdicarbollide metallacarboranes. For the reaction to proceed and avoid unreacted di-iodo starting material a high ratio (2.5:1) of the Grignard reagent per iodine in ...
... The synthesis of [3]--[6]- has shown that the modified Kumada reaction can be applied to produce B-C bonds for di-substituted cobaltabisdicarbollide metallacarboranes. For the reaction to proceed and avoid unreacted di-iodo starting material a high ratio (2.5:1) of the Grignard reagent per iodine in ...
Alcohols, Phenols, and Ethers
... possible number to the carbon bearing the hydroxyl group. • Step 3. Locate the position of the hydroxyl group by the number of the C to which it is attached. • Step 4. Locate and name any other substituents. • Step 5. Combine the name and location for other groups, the hydroxyl group location, and t ...
... possible number to the carbon bearing the hydroxyl group. • Step 3. Locate the position of the hydroxyl group by the number of the C to which it is attached. • Step 4. Locate and name any other substituents. • Step 5. Combine the name and location for other groups, the hydroxyl group location, and t ...
Alcohols, etc.
... with ol. The chain is numbered from the end giving the OH carbon the lower number. The name is prefixed with the number indicating the position of the OH group. For cyclic alcohols, the OH is at C-1. ...
... with ol. The chain is numbered from the end giving the OH carbon the lower number. The name is prefixed with the number indicating the position of the OH group. For cyclic alcohols, the OH is at C-1. ...
Derivatization - Sigma
... (e.g., O-H, COOH, N-H, and S-H) on these polar compounds determines the bonding and shape, type and strength of intermolecular forces, physical properties, and chemical reactivity of a molecule. Functional groups are less stable than the carbon backbone and are likely to participate in chemical reac ...
... (e.g., O-H, COOH, N-H, and S-H) on these polar compounds determines the bonding and shape, type and strength of intermolecular forces, physical properties, and chemical reactivity of a molecule. Functional groups are less stable than the carbon backbone and are likely to participate in chemical reac ...
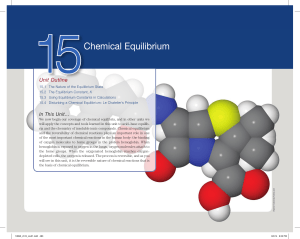
Chapter 15: Chemical Equilibrium
... In chemical equations, an equilibrium arrow () is used to indicate that a reaction is reversible. NO2(g) 1 CO(g) NO(g) 1 CO2(g) The reversibility of chemical reactions can be very useful in chemical synthesis, such as the conversion between alkenes and alcohols (Interactive Figure 15.1.1). Althou ...
... In chemical equations, an equilibrium arrow () is used to indicate that a reaction is reversible. NO2(g) 1 CO(g) NO(g) 1 CO2(g) The reversibility of chemical reactions can be very useful in chemical synthesis, such as the conversion between alkenes and alcohols (Interactive Figure 15.1.1). Althou ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.