C−C, C−O, C−N Bond Formation on sp2 Carbon by Pd(II)
... the bond susceptible to addition reactions. Many of these are catalyzed by palladium, perhaps the most versatile and widely used transition metal, which can exist in three easily interconvertible oxidation states: Pd(0), Pd(II), and Pd(IV). A wealth of reviews1 and books2 on organopalladium chemistr ...
... the bond susceptible to addition reactions. Many of these are catalyzed by palladium, perhaps the most versatile and widely used transition metal, which can exist in three easily interconvertible oxidation states: Pd(0), Pd(II), and Pd(IV). A wealth of reviews1 and books2 on organopalladium chemistr ...
SUPPORTED LIGANDS FOR METAL CATALYZED REACTIONS Rocío Marcos Escartín ISBN:
... Homogeneous metal catalysts are composed by a metal complex modified with organic ligands. Although the first non enzymatic asymmetric catalysts known were simple organic molecules,[1] the research regarding catalysis reached full development with metal-based systems, which have been predominant for ...
... Homogeneous metal catalysts are composed by a metal complex modified with organic ligands. Although the first non enzymatic asymmetric catalysts known were simple organic molecules,[1] the research regarding catalysis reached full development with metal-based systems, which have been predominant for ...
Sect 5 NMR Trends
... If J’s are same, then can use splitting (# of lines) = total # of H neighbors + 1 ...
... If J’s are same, then can use splitting (# of lines) = total # of H neighbors + 1 ...
2007 Nov Paper 1 - A Level Tuition
... In graphite, each C atom is bonded covalently to three other carbon atoms, using three of the valence electrons. The fourth valence electron is available for π bonding between adjacent carbon atoms, resulting in delocalisation throughout the layer. Sodium has a giant metallic structure, which consis ...
... In graphite, each C atom is bonded covalently to three other carbon atoms, using three of the valence electrons. The fourth valence electron is available for π bonding between adjacent carbon atoms, resulting in delocalisation throughout the layer. Sodium has a giant metallic structure, which consis ...
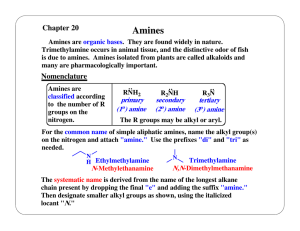
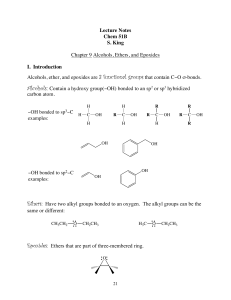
Alcohols, Phenols, and Ethers
... Lower molecular weight alcohols are soluble in water. This is due to hydrogen bonding between hydroxy group and water. ...
... Lower molecular weight alcohols are soluble in water. This is due to hydrogen bonding between hydroxy group and water. ...
Chapter #14 Newest CD
... CnH2n+2 Compounds containing only carbon and hydrogen with only single bonds and no multiple bonds, but a ring structure - Saturated hydrocarbons - Cycloalkanes CnH2n Compounds containing only carbon and hydrogen with double bonds - Unsaturated hydrocarbons - Alkenes CnH2n Compounds containing only ...
... CnH2n+2 Compounds containing only carbon and hydrogen with only single bonds and no multiple bonds, but a ring structure - Saturated hydrocarbons - Cycloalkanes CnH2n Compounds containing only carbon and hydrogen with double bonds - Unsaturated hydrocarbons - Alkenes CnH2n Compounds containing only ...
Transformation of Carbon Dioxide
... formation of a methyl carbonato complex through a reaction of tin methoxide with CO2 was reported in 1967.48,49 The formation of DMC upon thermolysis of methyl (carbonato) tin was first reported in 1975, but the yield was very low; only 10% based on tin.50 Another group later reported similar result ...
... formation of a methyl carbonato complex through a reaction of tin methoxide with CO2 was reported in 1967.48,49 The formation of DMC upon thermolysis of methyl (carbonato) tin was first reported in 1975, but the yield was very low; only 10% based on tin.50 Another group later reported similar result ...
Equilibrium Reversible Reactions
... Are these the products or reactants? _____________________________________ In graph two which components start with a concentration of 0mol/L? _____________ Are these the products or reactants? _____________________________________ This shows that these two graphs represent the same chemical reactio ...
... Are these the products or reactants? _____________________________________ In graph two which components start with a concentration of 0mol/L? _____________ Are these the products or reactants? _____________________________________ This shows that these two graphs represent the same chemical reactio ...
Unit 3: 1 Equilibrium and the Constant, K
... have very large versus very small concentrations at equilibrium. [See SP 2.2, 2.3; Essential knowledge 6.A.4] Learning objective 6.8 The student is able to use Le Chatelier’s principle to predict the direction of the shift resulting from various possible stresses on a system at chemical equilibrium. ...
... have very large versus very small concentrations at equilibrium. [See SP 2.2, 2.3; Essential knowledge 6.A.4] Learning objective 6.8 The student is able to use Le Chatelier’s principle to predict the direction of the shift resulting from various possible stresses on a system at chemical equilibrium. ...
Proofs to - Research Explorer
... alkoxycarbene is the promotion of an intramolecular process involving the formation of a hydroxyvinylidene intermediate [M{=C=CH(CH2)nOH}]+ which subsequently undergoes intramolecular addition at Cα to give a cyclic oxacarbene product [27, 29-31]. This method has proved to be effective even at the e ...
... alkoxycarbene is the promotion of an intramolecular process involving the formation of a hydroxyvinylidene intermediate [M{=C=CH(CH2)nOH}]+ which subsequently undergoes intramolecular addition at Cα to give a cyclic oxacarbene product [27, 29-31]. This method has proved to be effective even at the e ...
A review of new developments in the Friedel–Crafts - Beilstein
... the need for more environmentally and economically benign processes, the development of FC reactions using only catalytic amounts of a metal or acid catalyst would be highly desirable. In addition, the substitution of the alkyl chlorides by other, less toxic, alkylating reagents such as alcohols wou ...
... the need for more environmentally and economically benign processes, the development of FC reactions using only catalytic amounts of a metal or acid catalyst would be highly desirable. In addition, the substitution of the alkyl chlorides by other, less toxic, alkylating reagents such as alcohols wou ...
VITA - Trace: Tennessee Research and Creative Exchange
... thought to be the reaction intermediate. Yet the transfer of a nitrene from an imido complex is a possible target for a potential reaction intermediate. Upon examining isolated late transition metal imido complexes it becomes apparent that strong σ-donor mono-, bi-, and tridentate ligands can be use ...
... thought to be the reaction intermediate. Yet the transfer of a nitrene from an imido complex is a possible target for a potential reaction intermediate. Upon examining isolated late transition metal imido complexes it becomes apparent that strong σ-donor mono-, bi-, and tridentate ligands can be use ...
Full Text
... group should selectively react in good yield to give a protected substrate and should be selectively removed in good yield by readily available, preferably nontoxic reagents that do not attack the regenerated functional group.1 One of the most abundant functional groups is the hydroxyl group, which ...
... group should selectively react in good yield to give a protected substrate and should be selectively removed in good yield by readily available, preferably nontoxic reagents that do not attack the regenerated functional group.1 One of the most abundant functional groups is the hydroxyl group, which ...
Chm 2
... ____ 59. What is the name of a list of elements arranged according to the ease with which they undergo certain chemical reactions? a. reactivity list c. activity series b. reaction sequence d. periodic list ____ 60. An element in the activity series can replace any element a. in the periodic table. ...
... ____ 59. What is the name of a list of elements arranged according to the ease with which they undergo certain chemical reactions? a. reactivity list c. activity series b. reaction sequence d. periodic list ____ 60. An element in the activity series can replace any element a. in the periodic table. ...
Chapter 4 Alcohols and Alkyl Halides
... Alcohols and Alkyl Halides Alcohols and alkyl halides are very important functional groups. A functional group is an atom or group of atoms that undergoes certain reactions that are typical of that functional group. It is important to recognize functional groups since it makes the organization and l ...
... Alcohols and Alkyl Halides Alcohols and alkyl halides are very important functional groups. A functional group is an atom or group of atoms that undergoes certain reactions that are typical of that functional group. It is important to recognize functional groups since it makes the organization and l ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.












![[Ru(Triphos)H2(CO)] Characterisation - Durham e](http://s1.studyres.com/store/data/017676948_1-4352644236c53cc416f065328f560d26-300x300.png)