* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sect 5 NMR Trends
Survey
Document related concepts
Transcript
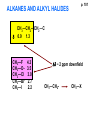
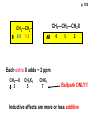
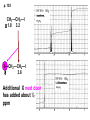
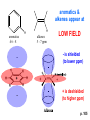
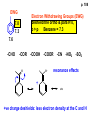
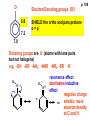
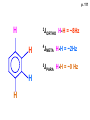
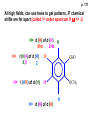
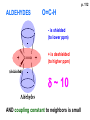
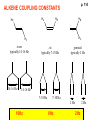
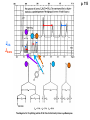
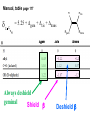
ORGANIC NMR INTERPRETATION p. 101 ALKANES AND ALKYL HALIDES d p. 101 CH3—CH2—CH2—C 0.9 1.3 CH3—F 4.3 CH3—O- 3.5 CH3—Cl 3.0 CH3—Br 2.7 CH3—I 2.2 Dd ~ 2 ppm downfield CH3—CH2- CH3—X p. 102 Inductive effects d CH3—CH2—CH2—C 0.9 1.3 d CH3—CH2—CH2Cl 1.0 1.8 3.5 CH3—CH2—CH2Br d 1.0 1.8 3.4 CH3—CH2—CH2I d 1.0 1.8 3.2 Dd 0 ½ 2 Effect falls off with distance and is ~ 0 two C away p. 102 CH3—CH2—CH2Cl 1.0 1.8 3.5 CH3—CH2—CH2Br 1.0 1.8 3.4 CH3—CH2—CH2I 1.0 1.8 3.2 Dd 0 ½ 2 d CH3—CH2— 0.9 1.3 p. 103 d CH3—CH2— 0.9 1.3 CH3—CH2—CH2X Dd 0 ½ 2 Each extra X adds ~ 2 ppm CH3—X d 3 CH2X2 5 CHX3 7 Ballpark ONLY!! Inductive effects are more or less additive p. 103 CH3—CH2—I d 1.8 3.2 I—CH2—CH2—I d 3.6 Additional X next door has added about ½ ppm p. 103 In general, the more substituted, the more downfield CH3—I d 2.2 CH3—CH2—I 3.2 (CH3)2CH—I 4.2 but additional alkyl groups are not as strong as –X CH3CH2Br = 3.4 (CH3)2CHBr =4.3 split by 6 = 6+1 6H 1H CH3CHBr2 = 5.5 split by 1 = 1+1 I—CH(CH3)2 p. 104 More complex splittings I—CH2—CH2—CH2—CH3 t ? ? t What about when neighbors are chemically different? If J’s are same, then can use splitting (# of lines) = total # of H neighbors + 1 3 2 1 H 3 H J=7 4 H J=7 J=0 Characteristic chain splitting in alkane chains More complex splittings I—CH2—CH2—CH2—CH3 t 5 2+2+1 6 t 2+3+1 p. 104 p. 105 ANISOTROPIC EFFECTS Spherical atoms have same effect in all directions F p-electrons H H H H are above and below the plane of molecule so electron density is different above or below molecule than in plane p. 105 so alkenes and aromatics (and other p-bonds) are not isotropic – they have effects that are different in different directions – we call them ANISOTROPIC Circulation of p the -electrons around the ring produce a field, Blocal = Bp-electrons = Blocal Bo + Blocal H H (deshielded) increased chemical shift in the plane of the ring Bo - Blocal Bo (shielded) decreased chemical shift above and below the plane of the ring so H feels B0 + Blocal so appear at low field aromatics & alkenes appear at H aromatics d6-8 - H alkenes 5 - 7 ppm LOW FIELD - is shielded (to lower ppm) + - + is deshielded (to higher ppm) p. 105 aromatic hydrogens ~ d 7 p. 106 7.2 2.3 7.0 2.3 6.8 2.3 X=C—CH3 methyl on an aromatic ring, double bond or carbonyl ~2.3 p. 107 Ph—CH2—CH2—(CH2)4—CH2—CH3 7.1-7.3 2.6 1.6 1.3 1.3 0.9 Ph—CH2—CH2—(CH2)6—CH2—CH3 p. 107 Clearing up some terminology: Downfield Deshielded Low field Greater d Upfield Shielded High field Smaller d p. 108 EWG Electron Withdrawing Groups (EWG) deshield the ortho & para H’s, o>p Benzene = 7.3 7.8 7.3 7.6 -CHO -COR -COOH -COOR O- O -CN -NO2 -SO2 resonance effects + etc +ve charge deshields: less electron density at the C and H 2:1:2 d 10 CHO EWG deshield 7.8 7.3 7.6 p. 108 p. 109 D Electron Donating groups (D:) 6.8 7.2 SHIELD the ortho and para protons o>p 7.0 Donating groups are X: (atoms with lone pairs but not halogens) e.g. -OH -OR -NH2 -NHR -NR2 -SR -R R R O: - O+ resonance effect dominates inductive effect negative charge shields: more etc electron density at C and H p. 109 OCH3 6.8-6.9 7.2 6.9-7.0 CH3 on O + Ar ring ~ 3.8 HALOGENS p. 110 Not easy to predict: Lone pairs shield by resonance but deshield because of high electronegativity Part of Table on manual page 110 Increments add to d 7.27 to predict shifts, e.g. Proton ortho to –CHO will be 7.27 + 0.58 = 7.85 O H p. 110 C AH HA BH HB OCH3 p-anisaldehyde d = 7.27 + 0.58 (ortho CHO) - 0.10 (meta OCH3) d = 7.75 ppm predicted shift of HA d = 7.27 - 0.46 (ortho OCH3) + 0.20 (meta CHO) d = 7.01 ppm predicted shift of HB p. 110 Look carefully at peaks, they are doublets CHO CH3O H1 H2 3J What about to other protons? ~ 8 Hz p. 111 H 3J ORTHO H H H H-H = ~8Hz 4J META H-H = ~2Hz 5J PARA H-H = ~0 Hz p. 111 At high fields, can use trees to get patterns, IF chemical shifts are far apart (called 1st order spectrum if Dd >> J) H= d (H) of d (H) H 8Hz 2Hz H= t (HH) of d (H) 8,8 2 H CHO H= t (HH) of d (H) H OCH3 H H= d (H) of d (H) p. 112 ALDEHYDES O=C-H - is shielded (to lower ppm) + is deshielded (to higher ppm) d ~ 10 AND coupling constant to neighbors is small H2C 3 JHH ~ 1 Hz a C H O Karplus showed relationship of J and a p. 112 p. 113 60MHz 60Hz Spectrum looks different on different instruments 30Hz J in Hertz is always independent of field p. 114 ALKENE COUPLING CONSTANTS HB HB HA HA HC HC trans typically 14-16 Hz 14-16 Hz cis typically 7-10 Hz geminal typically 2 Hz 14-16 Hz 7-10 Hz 7-10 Hz 2 Hz 2 Hz 16Hz 8Hz 2Hz mono-substituted alkene R--CH=CH2 p. 114 Always 12 lines: d(JL)d(JM) d(JL)d(JS) d(JM)d(JS) p. 115 Jcis Jtrans p. 116 Chemical shifts – much like aromatics p. 117 ‘normal’ = 5.25 ppm d+ E=W d- H C C proton is deshielded, signal shifts downfield to higher ppm d- .. ED H d+ C C proton is shielded, signal shifts upfield to lower ppm b to the substituent feels resonance effect Geminal (same C) always deshield by ~ 1ppm Manual, table page 117 H dC Rcis = 5.25 + Dgem + Dcis + Dtrans C H Always deshield geminal Shield b Rgem Deshield b Rtrans 4J = ~1Hz H1 4J = ~1Hz H 2 H H 4 3 3 2 1 p. 118 H 3J = ~7Hz Cis Me Gem H Trans Ph H d cis to Me = 5.25 + 0 - 0.22 – 0.07 = 4.96 d cis to Ph = 5.25 + 0 + 0.36 – 0.28 = 5.33 Cis Ph Trans Me H H See yellow pages A5 ALCOHOLS, AMINES, AMIDES AND ACIDS – exchangeable H’s R OH Ar O OH Ar-CONHR and Ar-CONH2 R NH2 Ar d 1-5 R C OH NH2 d 5-10 d 10-16 Hydrogen is becoming more acidic. More acidic More acidic = better able to form hydrogen bonds. means more Shifts further downfield (higher ppm). d+ on H [H3O+] + [-OH] 2 H2O ROH + R*OH' [ROHH']+ + [R*O]- Just like water, alcohols are always swapping their hydrogen partners. This happens rapidly: 105 times per second at room temperature. NMR time scale is ~ 10-2 – 10-3 sec fastest NMR can measure! p. 119 p. 119 Since acidic H exchange between molecules occurs faster than the NMR time scale they DO NOT show coupling to any neighbours and are typically broadened p. 119 To prove which is –OH peak, add D2O and shake ROH + D2O => ROD + HOD d ~ 5.2 Shape of peak depends upon temperature (rate of exchange is affected by temperature) exchange stopped Coupling visible slow exchange fast exchange HO—CH3 p. 120 p. 121 Only a triplet Amines: RNH2 d 1-5; ArNH2 d 5-10 p. 121 Amides: d 5-10 O- O Why 2? + R NH2 R N H H p. 121 Acids: d 10-16 d = 9.85 + offset (2.0) = 11.85 You can now start Assignment 5