Limiting Reactants and Percentage Yield
... Place the balls on an ungreased cookie sheet. 5. Bake at 350 °F for about 10 minutes, or until the cookies are light brown. ...
... Place the balls on an ungreased cookie sheet. 5. Bake at 350 °F for about 10 minutes, or until the cookies are light brown. ...
Physical Sciences Grade 12 Term 2
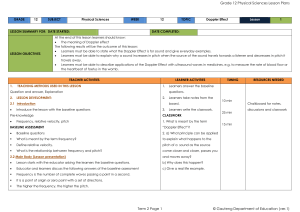
... The siren of a burglar alarm system has a frequency of 960 Hz. During a patrol, a security officer, travelling in his car, hears the siren of the alarm of a house and approaches the house at 2.1 Name the phenomenon that explains the change in the observed frequency. 2.2 Calculate the speed at which ...
... The siren of a burglar alarm system has a frequency of 960 Hz. During a patrol, a security officer, travelling in his car, hears the siren of the alarm of a house and approaches the house at 2.1 Name the phenomenon that explains the change in the observed frequency. 2.2 Calculate the speed at which ...
infrared spectroscopy
... C-H stretch occurs in region of 3095 – 3010 cm-1 (note higher wavenumber relative to alkanes) C=C stretch occurs in region of 1670 – 1640 cm-1 Can be used to determine type of substitution: Symmetrically substituted does not absorb at all A cis isomer absorbs more strongly than a trans isomer (cis i ...
... C-H stretch occurs in region of 3095 – 3010 cm-1 (note higher wavenumber relative to alkanes) C=C stretch occurs in region of 1670 – 1640 cm-1 Can be used to determine type of substitution: Symmetrically substituted does not absorb at all A cis isomer absorbs more strongly than a trans isomer (cis i ...
HYBRID MULTIDENTATE PHOSPHINE
... The coordination chemistry of four ligands (17, 32, 33 and 46) with various transition metals (Pt, Pd, Cu, Rh and Au) has been investigated in a comprehensive spectroscopis study. Single crystal X-ray analysis has been conducted at suitable junctures within the project. A surprising finding was that ...
... The coordination chemistry of four ligands (17, 32, 33 and 46) with various transition metals (Pt, Pd, Cu, Rh and Au) has been investigated in a comprehensive spectroscopis study. Single crystal X-ray analysis has been conducted at suitable junctures within the project. A surprising finding was that ...
amine
... Amides (RCONH2) are nonbasic • Amides do not undergo substantial protonation when treated with acids • Amides are poor nucleophiles • Nitrogen lone-pair electrons are stabilized through orbital overlap with the carbonyl group ...
... Amides (RCONH2) are nonbasic • Amides do not undergo substantial protonation when treated with acids • Amides are poor nucleophiles • Nitrogen lone-pair electrons are stabilized through orbital overlap with the carbonyl group ...
CHAPTER 9 Notes
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
APPROACHES TO CARBOHYDRATE-BASED CHEMICAL LIBRARIES: THE
... these potential benefits, the search for superior new leads is sufficient to justify an investment in combinatorial chemistry. Nevertheless, this stated purpose is artificially limiting and presents a somewhat myopic view of the potential of combinatorial chemistry. The methodical process of refini ...
... these potential benefits, the search for superior new leads is sufficient to justify an investment in combinatorial chemistry. Nevertheless, this stated purpose is artificially limiting and presents a somewhat myopic view of the potential of combinatorial chemistry. The methodical process of refini ...
IUPAC System of Nomenclature
... two differences. The parent chain must include the double bond even if it makes it shorter than the others. And the parent alkene chain must be numbered from whichever end gives the first carbon of the double bond the lower of two possible numbers. Also, the location number should be given as to whe ...
... two differences. The parent chain must include the double bond even if it makes it shorter than the others. And the parent alkene chain must be numbered from whichever end gives the first carbon of the double bond the lower of two possible numbers. Also, the location number should be given as to whe ...
History of Organic Chemistry
... A student is told to draw the structural diagram of the compound with formula C4H10 . Confused, the student tells the naive chemistry-challenged person that the task is impossible. a) Why can=t the student show the structure of C4H10? (2) b) Rephrase the question by changing one word so that the que ...
... A student is told to draw the structural diagram of the compound with formula C4H10 . Confused, the student tells the naive chemistry-challenged person that the task is impossible. a) Why can=t the student show the structure of C4H10? (2) b) Rephrase the question by changing one word so that the que ...
Document
... A) The concentration of N2will increase B) The concentration of H2will decrease C) The concentration of NH3will decrease D) The concentration of both N2and H2will increase ...
... A) The concentration of N2will increase B) The concentration of H2will decrease C) The concentration of NH3will decrease D) The concentration of both N2and H2will increase ...
Chapter 4 - AP Chemistry with dr hart
... you had better carry it out in the hood, or you will be very unpopular! • But just as in the previous examples, a gas is formed as a product of this reaction. Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Aqueous Reactions © 2009, Prentice-Hall, Inc. ...
... you had better carry it out in the hood, or you will be very unpopular! • But just as in the previous examples, a gas is formed as a product of this reaction. Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Aqueous Reactions © 2009, Prentice-Hall, Inc. ...
Alcohols, Phenols and Ethers
... 44) As the molar mass of these alcohols increases, the water solubility decreases. This occurs because the polarity of the hydroxyl group, which is the reason for the interaction with the polar water molecules, becomes less important as the size of the nonpolar hydrocarbon portion of the molecule in ...
... 44) As the molar mass of these alcohols increases, the water solubility decreases. This occurs because the polarity of the hydroxyl group, which is the reason for the interaction with the polar water molecules, becomes less important as the size of the nonpolar hydrocarbon portion of the molecule in ...
Atmospheric Formation_TELTEK
... 2.2 (CH3)2NH Atkinson et al.19 studied the kinetics of the OH radical reaction with (CH3)2NH over the temperature range 299 – 426 K and reported a negative Arrhenius activation energy, kOH(T) = 2.89×10-11 × exp{(245 ± 150)K/T} and kOH = (6.54 ± 0.66) × 10-11 cm3 molecule-1 s-1 at 298 K. Carl and Cro ...
... 2.2 (CH3)2NH Atkinson et al.19 studied the kinetics of the OH radical reaction with (CH3)2NH over the temperature range 299 – 426 K and reported a negative Arrhenius activation energy, kOH(T) = 2.89×10-11 × exp{(245 ± 150)K/T} and kOH = (6.54 ± 0.66) × 10-11 cm3 molecule-1 s-1 at 298 K. Carl and Cro ...
haloalkanes and arenes
... electronegative than the latter. Therefore, the density of electrons of C−Cl bond near the Clatom is less in chlorobenzene than in cydohexyl chloride. Moreover, the −R effect of the benzene ring of chlorobenzene decreases the electron density of the C−Cl bond near the Cl-atom. As a result, the polar ...
... electronegative than the latter. Therefore, the density of electrons of C−Cl bond near the Clatom is less in chlorobenzene than in cydohexyl chloride. Moreover, the −R effect of the benzene ring of chlorobenzene decreases the electron density of the C−Cl bond near the Cl-atom. As a result, the polar ...
Study Guide Chapter 4 Alcohols and Alkyl Halides
... proportions of hydrogen atom removal are given by the product of the statistical distribution and the relative rate per hydrogen. Given that a secondary hydrogen is abstracted 3.9 times faster than a primary one, we write the expression for the amount of chlorination at the primary relative to that ...
... proportions of hydrogen atom removal are given by the product of the statistical distribution and the relative rate per hydrogen. Given that a secondary hydrogen is abstracted 3.9 times faster than a primary one, we write the expression for the amount of chlorination at the primary relative to that ...
Modern Synthetic Methods for Copper-Mediated C(aryl
... detail the remarkable simplicity of the new reaction conditions. A mixture of the phenol (1 equiv), aryl boronic acid (2–3 equiv), anhydrous Cu(OAc)2 (1–2 equiv), and Et3N (2– 3 equiv) in dichloromethane were stirred at room temperature for 1–2 days and then the product was isolated in good yield af ...
... detail the remarkable simplicity of the new reaction conditions. A mixture of the phenol (1 equiv), aryl boronic acid (2–3 equiv), anhydrous Cu(OAc)2 (1–2 equiv), and Et3N (2– 3 equiv) in dichloromethane were stirred at room temperature for 1–2 days and then the product was isolated in good yield af ...
Unit 5: Oragnic Chemistry Notes (answers)
... 1. Most Alkenes and Alkynes are Non-Polar. They only have London Dispersion Forces. 2. Their boiling points are comparable to alkanes. The double bonds and triple bonds do NOT have any effect on their physical properties. 3. They do give off more heat (more exothermic) when burned (combusted) compar ...
... 1. Most Alkenes and Alkynes are Non-Polar. They only have London Dispersion Forces. 2. Their boiling points are comparable to alkanes. The double bonds and triple bonds do NOT have any effect on their physical properties. 3. They do give off more heat (more exothermic) when burned (combusted) compar ...
Ans:- (i) Gluconic acid - Kendriya Vidyalaya No.2, Kribhco, Surat
... Q12. A and B liquids on mixing produced a warm solution. Which type of deviation is there and why? Ans: Negative type of deviation is present. In the negative deviation the solute-solution (A-A) interaction and solvent-solvent (B-B) interaction will be weaker than solute-solvent(A-B ) interaction. S ...
... Q12. A and B liquids on mixing produced a warm solution. Which type of deviation is there and why? Ans: Negative type of deviation is present. In the negative deviation the solute-solution (A-A) interaction and solvent-solvent (B-B) interaction will be weaker than solute-solvent(A-B ) interaction. S ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.