Aldehydes and Ketones
... NAMING ALDEHYDES • ALDEHYDES ARE NAMED BY REPLACING THE FINAL “E” OF THE NAME OF THE ALKANE WITH THE SAME NUMBER OF CARBONS TO “AL”. • BECAUSE IN ALDEHYDES THE CARBONYL GROUP IS ALWAYS ATTACHED TO THE FIRST CARBON, THERE IS NO NEED TO PLACE A 1 IN FRONT OF THE NAME. • IF THERE ARE SUBSTITUENTS PRES ...
... NAMING ALDEHYDES • ALDEHYDES ARE NAMED BY REPLACING THE FINAL “E” OF THE NAME OF THE ALKANE WITH THE SAME NUMBER OF CARBONS TO “AL”. • BECAUSE IN ALDEHYDES THE CARBONYL GROUP IS ALWAYS ATTACHED TO THE FIRST CARBON, THERE IS NO NEED TO PLACE A 1 IN FRONT OF THE NAME. • IF THERE ARE SUBSTITUENTS PRES ...
Aldehydes and Ketones
... Aldehydes (RCHO) and ketones (R2CO) are characterized by the the carbonyl functional group (C=O) The compounds occur widely in nature as intermediates in metabolism and biosynthesis ...
... Aldehydes (RCHO) and ketones (R2CO) are characterized by the the carbonyl functional group (C=O) The compounds occur widely in nature as intermediates in metabolism and biosynthesis ...
Aldehydes and Ketones
... Aldehydes (RCHO) and ketones (R2CO) are characterized by the the carbonyl functional group (C=O) The compounds occur widely in nature as intermediates in metabolism and biosynthesis ...
... Aldehydes (RCHO) and ketones (R2CO) are characterized by the the carbonyl functional group (C=O) The compounds occur widely in nature as intermediates in metabolism and biosynthesis ...
C1_5_products_from_oils_crossword
... 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something that cannot be replaced once it is used up. 2. An alkene with the formula C2H4. 3. The reaction used in the oil industry to break ...
... 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something that cannot be replaced once it is used up. 2. An alkene with the formula C2H4. 3. The reaction used in the oil industry to break ...
Experiment 7-Reduction
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
orgchem rev integ odd numbers
... Inductive Effect – is an electronic effect due to the polarization of sigma bonds within a molecule or ion. Typically due to an electronegativity difference between the atoms at either end of the bond. ...
... Inductive Effect – is an electronic effect due to the polarization of sigma bonds within a molecule or ion. Typically due to an electronegativity difference between the atoms at either end of the bond. ...
Rxns of Alkynes
... b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
... b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
organometallic reagents
... A convergent synthesis of the same number of steps is preferable to a linear synthesis. ...
... A convergent synthesis of the same number of steps is preferable to a linear synthesis. ...
1. 4-methyl-4-octanol oxidizes to form a) 4-methyl-4
... 7. Which of the following will have the highest boiling point? a) 2-hexanone b) 2-hexanol c) hexanal d) hexane 8. What is the name of the reaction between an alcohol and an aldehyde? a) oxidation b) reduction c) addition d) none of the above 9. What determines if a molecule is a reducing sugar? a) ...
... 7. Which of the following will have the highest boiling point? a) 2-hexanone b) 2-hexanol c) hexanal d) hexane 8. What is the name of the reaction between an alcohol and an aldehyde? a) oxidation b) reduction c) addition d) none of the above 9. What determines if a molecule is a reducing sugar? a) ...
Organic Chemistry –Syllabus- one Semester Sackler faculty of
... Brønsted-Lowry Definition, conjugation acid-base pair, Ka ,pKa ,Kb, how to determine the position of acid-base reaction, The Effect of Structure on pKa, The Henderson–Hasselbalch Equation, A buffer Solution, Lewis Acids and Bases. Organic Compounds + Alkanes double bond equivalent, alkyl group, Nome ...
... Brønsted-Lowry Definition, conjugation acid-base pair, Ka ,pKa ,Kb, how to determine the position of acid-base reaction, The Effect of Structure on pKa, The Henderson–Hasselbalch Equation, A buffer Solution, Lewis Acids and Bases. Organic Compounds + Alkanes double bond equivalent, alkyl group, Nome ...
Acyl Anions Derived from Enol Ethers
... The normal disconnection pattern of a carboxylic acid with a Grignard reagent and carbon dioxide as SEs (path a) and a disconnection leading to a carboxyl synthon with an "unnatural" negative charge (path b). Cyanide ion can act as an SE of a negatively charged carboxyl synthon. Its reaction with R ...
... The normal disconnection pattern of a carboxylic acid with a Grignard reagent and carbon dioxide as SEs (path a) and a disconnection leading to a carboxyl synthon with an "unnatural" negative charge (path b). Cyanide ion can act as an SE of a negatively charged carboxyl synthon. Its reaction with R ...
Ch 19 Aldehydes and Ketones
... - Aldehydes are more reactive than ketones for both steric and electronic reasons. - First, the H creates less steric hindrance so that the carbonyl C is more accessible. - Second, an organic group provides e- donating induction which stabilizes the + carbonyl C and makes it less reactive. - Formal ...
... - Aldehydes are more reactive than ketones for both steric and electronic reasons. - First, the H creates less steric hindrance so that the carbonyl C is more accessible. - Second, an organic group provides e- donating induction which stabilizes the + carbonyl C and makes it less reactive. - Formal ...
Ethers, Sulfides, Epoxides
... Generally, the hemiacetals and acetals are only a minor component of an equilibrium mixture. In order to favor formation of acetals the carbonyl compound and alcohol is reacted with acid in the absence of water. Dry HCl) The acetals or hemiacetals maybe converted back to the carbonyl compound by tre ...
... Generally, the hemiacetals and acetals are only a minor component of an equilibrium mixture. In order to favor formation of acetals the carbonyl compound and alcohol is reacted with acid in the absence of water. Dry HCl) The acetals or hemiacetals maybe converted back to the carbonyl compound by tre ...
Aldehydes and ketones
... • Aldehydes and ketones are polar compounds • The carbonyl group is polar – The oxygen end is electronegative – Can hydrogen bond to water – Cannot form intermolecular hydrogen bond ...
... • Aldehydes and ketones are polar compounds • The carbonyl group is polar – The oxygen end is electronegative – Can hydrogen bond to water – Cannot form intermolecular hydrogen bond ...
Isomers
... Stereo isomers have the same structural formulas but they differ in their spatial arrangements. There are two types of stereoisomerism ...
... Stereo isomers have the same structural formulas but they differ in their spatial arrangements. There are two types of stereoisomerism ...
W19 Aldehydes ketones I
... reaction scheme of aldehydes and ketones nucleophilic addition AN to C=O group: ...
... reaction scheme of aldehydes and ketones nucleophilic addition AN to C=O group: ...
Chapter One: Molecular Structure
... Predict the products of reactions involving ethers and epoxides with common reagents. Predict the likelihood of carbon skeleton rearrangement under a given set of conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Re ...
... Predict the products of reactions involving ethers and epoxides with common reagents. Predict the likelihood of carbon skeleton rearrangement under a given set of conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Re ...
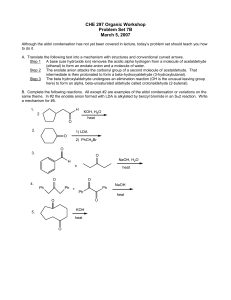
CHE 297 Organic Workshop
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
Chapter 6: Alkynes, reactions of alkynes, and multistep synthesis
... b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
... b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.