Reactions to functionalize benzene
... Due to the stability of aromatic system, addition reactions aren’t favored. Electrophilic aromatic substitution is the predominant reaction mechanism Hydrogens are easily replaced by electrophilic substituent groups H ...
... Due to the stability of aromatic system, addition reactions aren’t favored. Electrophilic aromatic substitution is the predominant reaction mechanism Hydrogens are easily replaced by electrophilic substituent groups H ...
What are reactions? - UTLNET Secure Site
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
What are reactions?
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
File
... Some chemical compounds have optical activity in the sense that these compounds have the ability to rotate the plane of polarized light. Polarized light has light waves all traveling parallel to each other. Ordinary light has light waves traveling in all directions. When polarized light is passed th ...
... Some chemical compounds have optical activity in the sense that these compounds have the ability to rotate the plane of polarized light. Polarized light has light waves all traveling parallel to each other. Ordinary light has light waves traveling in all directions. When polarized light is passed th ...
haloalkanes - Knockhardy
... alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for tertiary haloalkanes where there is steric hindrance to the attack and a more stable tert ...
... alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for tertiary haloalkanes where there is steric hindrance to the attack and a more stable tert ...
PowerPoint **
... a. Baylis-Hillman reaction: An acrylate ester reacts with an aldehyde in the presence of an amine or phosphine catalyst. ...
... a. Baylis-Hillman reaction: An acrylate ester reacts with an aldehyde in the presence of an amine or phosphine catalyst. ...
oigchem
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
... Which type of systems act as E+ or Nu-? Same system act as E+ or Nu- depending on which system it reacts. Acidity and Basicity: General idea of order of acidity and basicity. Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
CHEM1102 Worksheet 7: Reactions of Carbonyls and Acid
... Using the model above, predict the outcome of the following reactions. (Hint: LiAlH4 reacts as if it contains the hydride ion, H, and CH3CH2MgBr reacts as it contains the carbanion, CH3CH2) ...
... Using the model above, predict the outcome of the following reactions. (Hint: LiAlH4 reacts as if it contains the hydride ion, H, and CH3CH2MgBr reacts as it contains the carbanion, CH3CH2) ...
Lab Activity: Functional Groups
... 2. Place the two models next to each other so that the hydroxyl groups are facing. 3. Remove the hydroxyl group from one of the alcohols and the hydrogen atom from the hydroxyl group of the other alcohol. Now join the two molecules together. 4. Write down the structural equation for this reaction, n ...
... 2. Place the two models next to each other so that the hydroxyl groups are facing. 3. Remove the hydroxyl group from one of the alcohols and the hydrogen atom from the hydroxyl group of the other alcohol. Now join the two molecules together. 4. Write down the structural equation for this reaction, n ...
Nucleophilic Substitution Reaction
... Elimination Reactions Elimination reactions, in which two groups are removed from a molecule, not being replaced by another group, are the reverse of addition reactions. Usually they involve the loss of two substituents from vicinal atoms resulting in the formation of a double or triple bond. Most c ...
... Elimination Reactions Elimination reactions, in which two groups are removed from a molecule, not being replaced by another group, are the reverse of addition reactions. Usually they involve the loss of two substituents from vicinal atoms resulting in the formation of a double or triple bond. Most c ...
Enantioselective one-pot synthesis of dihydroquinolones via BINOL
... result either from intramolecular attack of an enolate onto an anilinederived imine, or alternatively from an initial Knoevenagel condensation followed by intramolecular 1,4-addition of the aniline. Treatment of α-benzyl substrate 28 with scandium triflate successfully generated product 29, suggesti ...
... result either from intramolecular attack of an enolate onto an anilinederived imine, or alternatively from an initial Knoevenagel condensation followed by intramolecular 1,4-addition of the aniline. Treatment of α-benzyl substrate 28 with scandium triflate successfully generated product 29, suggesti ...
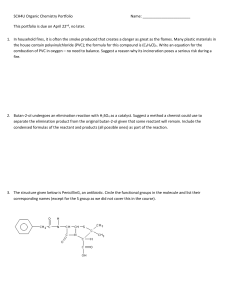
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... 1. In household fires, it is often the smoke produced that creates a danger as great as the flames. Many plastic materials in the house contain polyvinylchloride (PVC); the formula for this compound is (C2H3Cl)n. Write an equation for the combustion of PVC in oxygen – no need to balance. Suggest a r ...
... 1. In household fires, it is often the smoke produced that creates a danger as great as the flames. Many plastic materials in the house contain polyvinylchloride (PVC); the formula for this compound is (C2H3Cl)n. Write an equation for the combustion of PVC in oxygen – no need to balance. Suggest a r ...
Ch 23 Carbonyl Condensations
... - A diester can form stable ring with 5 or 6 atoms, such as with hexanedioate and heptanedioate esters. - The ring has the remaining CO2R on its #1 C and the (oxo) carbonyl on the #2 C. - Because the product is a -keto ester (like acetoacetate), it can be used in further substitutions. As in the ...
... - A diester can form stable ring with 5 or 6 atoms, such as with hexanedioate and heptanedioate esters. - The ring has the remaining CO2R on its #1 C and the (oxo) carbonyl on the #2 C. - Because the product is a -keto ester (like acetoacetate), it can be used in further substitutions. As in the ...
CHEM 202_ Part 2
... Carbonyl group is polar, so the compound may be attacked by a nucleophile or electrophile ...
... Carbonyl group is polar, so the compound may be attacked by a nucleophile or electrophile ...
chem 464l survival guide
... Both functional groups form hydrogen bonds readily via the hydrogen atoms (the hydrogen bond donor) and the oxygen or sulfur lone pairs (the hydrogen bond acceptors). Additionally, sulfhydryl (a.k.a. “thiol”) groups can perform some types of nucleophilic reactions in biochemistry as well as reductio ...
... Both functional groups form hydrogen bonds readily via the hydrogen atoms (the hydrogen bond donor) and the oxygen or sulfur lone pairs (the hydrogen bond acceptors). Additionally, sulfhydryl (a.k.a. “thiol”) groups can perform some types of nucleophilic reactions in biochemistry as well as reductio ...
Orbitals - drjosephryan.com
... ketones with hydride reagents may be represented as proceeding through a nucleophilic addition of a hydride ion (:H–) to the C=O carbon • LiAlH4 and NaBH4 act as if they are donors of hydride ion ...
... ketones with hydride reagents may be represented as proceeding through a nucleophilic addition of a hydride ion (:H–) to the C=O carbon • LiAlH4 and NaBH4 act as if they are donors of hydride ion ...
投影片 1
... The positive charge (+) is placed at the carbon attached to the E class function group (e.g.,=O,-OH, -Br) Consonant pattern: Positives charges are placed at carbon atoms bonded to the E class groups ...
... The positive charge (+) is placed at the carbon attached to the E class function group (e.g.,=O,-OH, -Br) Consonant pattern: Positives charges are placed at carbon atoms bonded to the E class groups ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.