* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Functional Groups - Madison County Schools
Survey
Document related concepts
Transcript
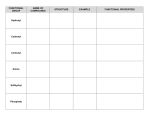
FUNCTIONAL GROUPS HYDROXYL GROUP: --OH • Polar, since O—H bond is polar covalent • Makes molecule water-soluble, with –OH forming hydrogen bonds • Organic compounds with hydroxyl groups are called ALCOHOLS. • Example: ethanol (C-C-OH) in alcoholic drinks CARBONYL GROUP: --CO • Functional group with a carbon double-bonded to an oxygen atom • Polar group, making molecules water-soluble • Functional group found in sugars. If carbonyl group is at the end of the carbon chain, the molecule is a Aldehyde, otherwise it is an Ketone. • Ex. Propanol (aldehyde) and acetone (ketone) CARBOXYL GROUP: --COOH • Group consists of a carbon atom doublebonded to an oxygen atom, and singlebonded to a hydroxyl group • Polar group, molecules are water-soluble • H+ can disassociate, giving the molecule acidic properties. • Compounds with group are called CARBOXYLIC ACIDS. • Ex. Acetic Acid (in vinegar) AMINO GROUP: --NH2 • Polar group that is water-soluble. • Acts as a weak base, accepting a proton (H+) • Organic compounds with group are called AMINES. • NOTE: Amino acids (monomers of proteins) have both an amino group and a carboxyl group SULFHYDRYL GROUP:--SH • Help stabilize the structure of proteins • Organic compounds with this functional group are THIOLS. • Ex. Cysteine • Sulfhydryl groups formed in hair during perms to give curly form PHOSPHATE GROUP: --PO4 • Dissociated form of H3PO4 • Has acidic properties since it loses H+ • Polar group, soluble in water • Compounds are ORGANIC PHOSPHATES, important in cellular energy storage and transfer (ATP). METHYL GROUP: --CH3 • Nonpolar, hydrophobic (all other functional groups are hydrophilic) • Important group in nonpolar amino acids, contributing to the tertiary structure of proteins • Compounds containing a methyl group are called methylated compounds