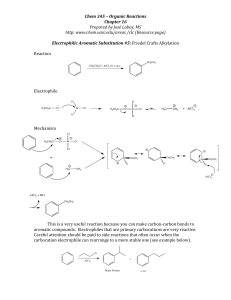
EAS Friedel-Crafts Alkylation
... The Friedel-‐Crafts reaction only requires catalytic amounts of the Lewis acid because it is recycled through the reaction. There is a major disadvantage of the alkylation reaction and that is over-‐ alkyla ...
... The Friedel-‐Crafts reaction only requires catalytic amounts of the Lewis acid because it is recycled through the reaction. There is a major disadvantage of the alkylation reaction and that is over-‐ alkyla ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... suffix -yl from the root of the carboxylic acid CH3CO: acetyl; CHO: formyl; C6H5CO: benzoyl; ArCO: aroyl The prefix oxo- is used if other functional groups are present ...
... suffix -yl from the root of the carboxylic acid CH3CO: acetyl; CHO: formyl; C6H5CO: benzoyl; ArCO: aroyl The prefix oxo- is used if other functional groups are present ...
ADDITION REACTIONS
... He found that, when two products were formed, one was formed in a larger quantity. His original rule was based only on this reaction. The modern version uses carbocation stability as a criterion for predicting the products. In the electrophilic addition to alkenes the major product is formed via the ...
... He found that, when two products were formed, one was formed in a larger quantity. His original rule was based only on this reaction. The modern version uses carbocation stability as a criterion for predicting the products. In the electrophilic addition to alkenes the major product is formed via the ...
Aldehydes and Ketones
... Nucleophilic Addition of Phosphorus Ylides: Using carbon Nucleophiles 3) The Wittig Reaction The Wittig reactions is an important method for the formation of alkanes The double bond forms specially at the location of the original aldehyde or ketone Ylides are naturel molecules but have +ve a ...
... Nucleophilic Addition of Phosphorus Ylides: Using carbon Nucleophiles 3) The Wittig Reaction The Wittig reactions is an important method for the formation of alkanes The double bond forms specially at the location of the original aldehyde or ketone Ylides are naturel molecules but have +ve a ...
CH 12-3 Power Point
... •The “Grignard Reagent” is essentially a carbon nucleophile in the form of a coordinate covalent bond. •The Grignard Reagent then reacts with an oxygen compound (aldehyde, ketone, ester, epoxide) to produce a new C-C bond and an alcohol. ...
... •The “Grignard Reagent” is essentially a carbon nucleophile in the form of a coordinate covalent bond. •The Grignard Reagent then reacts with an oxygen compound (aldehyde, ketone, ester, epoxide) to produce a new C-C bond and an alcohol. ...
Organic Chemistry
... Ans2. 2-Bromobutane contain one chiral C-atom. So, it is optically active, ...
... Ans2. 2-Bromobutane contain one chiral C-atom. So, it is optically active, ...
Origin of the Diastereoselection in the Indium
... where the phenyl group of benzaldehyde was placed in the equatorial position in both cases (Scheme 3). We assumed that the Z-configuration of the haloallylic sulfones 1 equilibrated to the more stable E-configuration while indium was being inserted. This was presumably the reason that the geometry o ...
... where the phenyl group of benzaldehyde was placed in the equatorial position in both cases (Scheme 3). We assumed that the Z-configuration of the haloallylic sulfones 1 equilibrated to the more stable E-configuration while indium was being inserted. This was presumably the reason that the geometry o ...
aldehyde ketone
... Due to the polarity of the C=O bond, a permanent dipole moment exists in aldehydes and ketones (dipole-dipole forces). Thus, the MP/BP of aldehydes and ketones is mid-range – higher than that of alkanes or alkenes (London forces) but lower than that of alcohols (hydrogen bonds). Nomenclature – IUPAC ...
... Due to the polarity of the C=O bond, a permanent dipole moment exists in aldehydes and ketones (dipole-dipole forces). Thus, the MP/BP of aldehydes and ketones is mid-range – higher than that of alkanes or alkenes (London forces) but lower than that of alcohols (hydrogen bonds). Nomenclature – IUPAC ...
CM1121 - ORGANIC CHEMISTRY 1
... ADVANCED PLACEMENT TEST MODULE DESCRIPTION CM1121 - Organic Chemistry 1 This module is intended for students majoring in Chemistry and Applied Chemistry. It deals primarily with the basic principles to understand the structure and reactivity of organic molecules. Emphasis is on substitution and elim ...
... ADVANCED PLACEMENT TEST MODULE DESCRIPTION CM1121 - Organic Chemistry 1 This module is intended for students majoring in Chemistry and Applied Chemistry. It deals primarily with the basic principles to understand the structure and reactivity of organic molecules. Emphasis is on substitution and elim ...
Exam - Chemistry With BT
... Give the major products for the reactions below. If the major product is a mixture of stereoisomers, show all the stereoisomers (in a way they can be distinguished from one another). ...
... Give the major products for the reactions below. If the major product is a mixture of stereoisomers, show all the stereoisomers (in a way they can be distinguished from one another). ...
R-c-H+H-oH:n-J-u oo o il o o o I o
... 7. One mole of methanolwould releasemore energy upon complete oxidation than one mole of methane. B. Both aldehydesand ketones have a carbonyl group. 9. All aldehydesand ketones give a positive Tollens' test. 10. Fourpairs ofelectrons are sharedinthe carbonyl bond formed between an oxygen and a carb ...
... 7. One mole of methanolwould releasemore energy upon complete oxidation than one mole of methane. B. Both aldehydesand ketones have a carbonyl group. 9. All aldehydesand ketones give a positive Tollens' test. 10. Fourpairs ofelectrons are sharedinthe carbonyl bond formed between an oxygen and a carb ...
Recall
... The next slides recall the diversity of nucleophiles that may be used. Observe that there is limited opportunity of creating new C-C bonds, welding together two R groups. We seem to be somewhat lacking in simple carbon based nucleophiles. ...
... The next slides recall the diversity of nucleophiles that may be used. Observe that there is limited opportunity of creating new C-C bonds, welding together two R groups. We seem to be somewhat lacking in simple carbon based nucleophiles. ...
Review sheet - Paws.wcu.edu.
... can add e- density by induction (-alkyl groups) can add e- density by resonance (-OH, -OR, -NR2, -Ph ) Deactivators: remove electron density from aromatic ring, reduce rate of EAS, direct meta can remove e- density by induction (-CF3, -N+R3, -SO3H, -NO2 ) can remove e- density by resonance (-NO2, -C ...
... can add e- density by induction (-alkyl groups) can add e- density by resonance (-OH, -OR, -NR2, -Ph ) Deactivators: remove electron density from aromatic ring, reduce rate of EAS, direct meta can remove e- density by induction (-CF3, -N+R3, -SO3H, -NO2 ) can remove e- density by resonance (-NO2, -C ...
Blank Final Exam from 2004 - Department of Chemistry | Oregon
... (A) Alanine (Ala) and Glycine (Gly) are amino acids both of which have 1 chiral carbon (B) a chiral molecule is not superimposable on its mirror image (C) the following molecule is chiral: O ...
... (A) Alanine (Ala) and Glycine (Gly) are amino acids both of which have 1 chiral carbon (B) a chiral molecule is not superimposable on its mirror image (C) the following molecule is chiral: O ...
16.1 The Carbonyl Group
... • Carbonyl compound: Any compound that contains a carbonyl group, C=O. • Carbonyl group: A functional group that has a C atom joined to an O atom by a double bond. • The bond angles between the three substituents on the carbonyl carbon atom are 120°, or close to it. ...
... • Carbonyl compound: Any compound that contains a carbonyl group, C=O. • Carbonyl group: A functional group that has a C atom joined to an O atom by a double bond. • The bond angles between the three substituents on the carbonyl carbon atom are 120°, or close to it. ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.