Reaction Rates/Chemical Kinetics
... • The relative orientations of the molecules during their collisions determine whether the atoms are suitably positioned to form new bonds. • Particles lacking necessary kinetic energy to react bounce apart when they collide. • Imagine clay balls… • The minimum amount of energy that particles must h ...
... • The relative orientations of the molecules during their collisions determine whether the atoms are suitably positioned to form new bonds. • Particles lacking necessary kinetic energy to react bounce apart when they collide. • Imagine clay balls… • The minimum amount of energy that particles must h ...
FUNCTIONAL GROUP IDENTIFICATION WORKSHEET
... Be able to identify and name alcohols. Be able to identify and name ethers. ...
... Be able to identify and name alcohols. Be able to identify and name ethers. ...
Chapter 23 SG5e
... nitration reaction. This leads to the desired product with the hydroxy group in the 3 position. Step (1) Oxidation at a benzylic carbon (Section 20.6A) can be brought about using chromic acid to give benzoic acid. Step (2) Nitration of the aromatic ring using HNO3 in H2SO4. The meta-directing carbox ...
... nitration reaction. This leads to the desired product with the hydroxy group in the 3 position. Step (1) Oxidation at a benzylic carbon (Section 20.6A) can be brought about using chromic acid to give benzoic acid. Step (2) Nitration of the aromatic ring using HNO3 in H2SO4. The meta-directing carbox ...
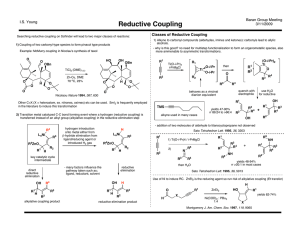
Reductive Couplings
... Development of NHC Ligands (allow for efficient intermoleuclar RC with triethylsilanes) ...
... Development of NHC Ligands (allow for efficient intermoleuclar RC with triethylsilanes) ...
Class Notes
... Super Strong Bases and Nucleophiles • The counterion metal is a spectator • Stability-reactivity principle: very unstable à very reactive • This great reactivity is very useful (as nucleophile) • This great reactivity (as base) has implication for proper technical use (see following) 7. Solvent and ...
... Super Strong Bases and Nucleophiles • The counterion metal is a spectator • Stability-reactivity principle: very unstable à very reactive • This great reactivity is very useful (as nucleophile) • This great reactivity (as base) has implication for proper technical use (see following) 7. Solvent and ...
One Step Formation of Propene from Ethene or Ethanol through
... complexes. At the moment, no reports claim nickel ion as a catalytically active species for metathesis. It is noteworthy that the surface density of Ni is approximately 0.5 Ni/nm2 in the case of Ni-M41(20) on the assumption of the even distribution of nickel on the surface. The valence of nickel ion ...
... complexes. At the moment, no reports claim nickel ion as a catalytically active species for metathesis. It is noteworthy that the surface density of Ni is approximately 0.5 Ni/nm2 in the case of Ni-M41(20) on the assumption of the even distribution of nickel on the surface. The valence of nickel ion ...
Follow Along Notes - Jackson County School System
... Which of the following statements about the reaction quotient, Q is false? a. The value of Q can be used to predict equilibrium concentrations. b. It has the same expression as Kc. c. Its value is calculated using nonequilibrium concentrations. d. If Q > Kc, the reaction must move to equilibrium by ...
... Which of the following statements about the reaction quotient, Q is false? a. The value of Q can be used to predict equilibrium concentrations. b. It has the same expression as Kc. c. Its value is calculated using nonequilibrium concentrations. d. If Q > Kc, the reaction must move to equilibrium by ...
carboxylic acids and their derivatives
... elimination, substitution, and rearrangement by polar, radical, and concerted mechanisms. Indeed, if you have been looking for similarities, you will have seen that most of the reactions discussed in the preceding three chapters are variations on basic types we have discussed earlier. Furthermore, m ...
... elimination, substitution, and rearrangement by polar, radical, and concerted mechanisms. Indeed, if you have been looking for similarities, you will have seen that most of the reactions discussed in the preceding three chapters are variations on basic types we have discussed earlier. Furthermore, m ...
II. Main types of organometallic compounds
... aromatic, acyl in the formation of the M – C bonding, there is only one carbon atom directly bonding with metal atom, which is σ ligands, they typically provide a pair of σ electron as anion (formal charge - 1), and its coordination way is the terminal type ...
... aromatic, acyl in the formation of the M – C bonding, there is only one carbon atom directly bonding with metal atom, which is σ ligands, they typically provide a pair of σ electron as anion (formal charge - 1), and its coordination way is the terminal type ...
The Major Classes of Chemical Reactions
... do not conduct an electric current, these substances are called nonelectrolytes. Many other covalent substances, such as benzene (C6H6) and octane (C8H18), do not contain polar bonds, and these substances do not dissolve appreciably in water. A small, but very important, group of H-containing covale ...
... do not conduct an electric current, these substances are called nonelectrolytes. Many other covalent substances, such as benzene (C6H6) and octane (C8H18), do not contain polar bonds, and these substances do not dissolve appreciably in water. A small, but very important, group of H-containing covale ...
course structure
... Group Theory: Symmetry elements and symmetry operations, Algebraic Operators, Point groups and it's determination in various molecules. Matrix mathematics & Matrix representation symmetry operation. Definition of a Group, Subgroup, Abelian group, Cyclic group. Rearrangement Theorem, Group multiplica ...
... Group Theory: Symmetry elements and symmetry operations, Algebraic Operators, Point groups and it's determination in various molecules. Matrix mathematics & Matrix representation symmetry operation. Definition of a Group, Subgroup, Abelian group, Cyclic group. Rearrangement Theorem, Group multiplica ...
Richard R. Schrock - Nobel Lecture
... ering the likely synthetic difficulties associated with R being a 2,6-di-tbutylphenyl group, we settled on the 2,6-diisopropylphenyl group, a choice that in part was in response to a comment by K. B. Sharpless concerning the value of isopropyl groups in general versus t-butyl groups. (The 2,6-diisop ...
... ering the likely synthetic difficulties associated with R being a 2,6-di-tbutylphenyl group, we settled on the 2,6-diisopropylphenyl group, a choice that in part was in response to a comment by K. B. Sharpless concerning the value of isopropyl groups in general versus t-butyl groups. (The 2,6-diisop ...
ENZYMES
... • Enzymes are effected by the following factors: – Temperature – pH – Concentration of enzyme – Concentration of substrate ...
... • Enzymes are effected by the following factors: – Temperature – pH – Concentration of enzyme – Concentration of substrate ...
Klein, 2e
... substitution process. Examine the following example • The leaving group is good, but what about the nucleophile? • Draw a complete mechanism. Each step is an equilibrium. Which side will the equilibrium favor? • If the nucleophile were used as the solvent (a solvolysis reaction), would that shift th ...
... substitution process. Examine the following example • The leaving group is good, but what about the nucleophile? • Draw a complete mechanism. Each step is an equilibrium. Which side will the equilibrium favor? • If the nucleophile were used as the solvent (a solvolysis reaction), would that shift th ...
Protection (and Deprotection) of Functional Groups in Organic
... limited thermal stability, whose manufacture generally is based on multistep synthesis performed in the liquid phase and frequently involving protectiondeprotection steps. The use of blocking functions in organic synthesis, developed for nearly 100 years, makes more complex the entire synthetic plan ...
... limited thermal stability, whose manufacture generally is based on multistep synthesis performed in the liquid phase and frequently involving protectiondeprotection steps. The use of blocking functions in organic synthesis, developed for nearly 100 years, makes more complex the entire synthetic plan ...
The Process of Chemical Reactions
... wide range of velocities and thus a wide range of kinetic energies. If you were riding on a particle—in a gas, for example—you would be constantly colliding with other particles, speeding up or slowing down, and increasing or decreasing your kinetic energy. Sometimes you collide with a slow moving p ...
... wide range of velocities and thus a wide range of kinetic energies. If you were riding on a particle—in a gas, for example—you would be constantly colliding with other particles, speeding up or slowing down, and increasing or decreasing your kinetic energy. Sometimes you collide with a slow moving p ...
Compounds with Oxygen Atoms
... Compounds of phenol are the active ingredients in the essential oils of nutmeg, thyme, cloves, and vanilla. ...
... Compounds of phenol are the active ingredients in the essential oils of nutmeg, thyme, cloves, and vanilla. ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.