R - Evans - Harvard University
... The a-azido carboximide products have been shown to be versatile a-amino acid synthons that may be readily converted to n-amino acids as well as to N-protected a-amino acid derivatives. The racemization-free removal of the chiral auxiliary was achieved in high yield both by saponification and transe ...
... The a-azido carboximide products have been shown to be versatile a-amino acid synthons that may be readily converted to n-amino acids as well as to N-protected a-amino acid derivatives. The racemization-free removal of the chiral auxiliary was achieved in high yield both by saponification and transe ...
Lorell Thesis Final Version in PDF S
... finished. To my husband, Tito, who has given me the happiest days of my life and stood beside me in all the happy and stressful moments. To my daughter Lorelys Maia, she is my whole life and is always inspiring me how to be a better person overall. To my parents José and Betzaida, who always support ...
... finished. To my husband, Tito, who has given me the happiest days of my life and stood beside me in all the happy and stressful moments. To my daughter Lorelys Maia, she is my whole life and is always inspiring me how to be a better person overall. To my parents José and Betzaida, who always support ...
Chem 33 Lab - Santa Clara University
... so that each student has a workstation in a hood, two students per hood. This significant enhancement in safety protects everyone because many organic compounds are volatile and using a hood minimizes your exposure to fumes. Therefore, virtually 100% of your chemical work should be performed in a fu ...
... so that each student has a workstation in a hood, two students per hood. This significant enhancement in safety protects everyone because many organic compounds are volatile and using a hood minimizes your exposure to fumes. Therefore, virtually 100% of your chemical work should be performed in a fu ...
10 Haloalkanes and Haloarenes
... In the case of unsymmetrical alkenes, carbon atoms involved in double bond are non-equivalent, so the addition of HX in unsymmetrical alkene takes place according to Markownikoff’s rule. According to Markownikoff’s rule, “during addition of an unsymmetrical reagent across the double bond of an unsym ...
... In the case of unsymmetrical alkenes, carbon atoms involved in double bond are non-equivalent, so the addition of HX in unsymmetrical alkene takes place according to Markownikoff’s rule. According to Markownikoff’s rule, “during addition of an unsymmetrical reagent across the double bond of an unsym ...
Organic Reactions in Organised Media
... is almost always the case when immiscible hydrophilic and hydrophobic reagents are involved in the process. Due to the relatively small interfacial area of the two-phase systems that are formed, the rate of such reactions is low unless special measures are taken. Typical reactions facing such a prob ...
... is almost always the case when immiscible hydrophilic and hydrophobic reagents are involved in the process. Due to the relatively small interfacial area of the two-phase systems that are formed, the rate of such reactions is low unless special measures are taken. Typical reactions facing such a prob ...
aq - Byron High School
... zero (rule 4). Letting x equal the oxidation number of S, we have 2(+1) + x = 0. Thus, S has an oxidation number of –2. (b) Because this is an elemental form of sulfur, the oxidation number of S is 0 (rule 1). (c) Because this is a binary compound, we expect chlorine to have an oxidation number of – ...
... zero (rule 4). Letting x equal the oxidation number of S, we have 2(+1) + x = 0. Thus, S has an oxidation number of –2. (b) Because this is an elemental form of sulfur, the oxidation number of S is 0 (rule 1). (c) Because this is a binary compound, we expect chlorine to have an oxidation number of – ...
Naming Organic Compounds I
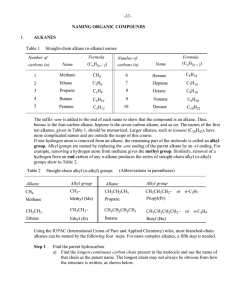
... N.B.: A complex substituent is alphabetized under the first letter of its name. Branched alkyl groups On page 40, it was shown that straight-chain alkyl (n-alkyl) groups are formed by removal of a terminal (end) hydrogen atom from straight-chain alkanes. It is also possible to generate a large numbe ...
... N.B.: A complex substituent is alphabetized under the first letter of its name. Branched alkyl groups On page 40, it was shown that straight-chain alkyl (n-alkyl) groups are formed by removal of a terminal (end) hydrogen atom from straight-chain alkanes. It is also possible to generate a large numbe ...
Water deuteration and ortho-to-para nuclear spin ratio of H2 in
... To simulate the formation and physical evolution of a molecular cloud, we use the one-dimensional shock model developed by B04 and H10. Here we briefly outline the model, while more details can be found in the original papers. The model describes the evolution of post-shock materials in a plane-para ...
... To simulate the formation and physical evolution of a molecular cloud, we use the one-dimensional shock model developed by B04 and H10. Here we briefly outline the model, while more details can be found in the original papers. The model describes the evolution of post-shock materials in a plane-para ...
REASONING QUESTIONS IN ORGANIC CHEMISTRY
... 36. Give plausible explanation for each of the following: (i) Cyclohexanone forms cyanohydrin in good yield but 2,2,6Trimethylcyclohexanone does not. (ii) There are two –NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones. (iii) During the preparation of est ...
... 36. Give plausible explanation for each of the following: (i) Cyclohexanone forms cyanohydrin in good yield but 2,2,6Trimethylcyclohexanone does not. (ii) There are two –NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones. (iii) During the preparation of est ...
amines
... A nitrogen that bears four substituents is positively charged and is named as an ammonium ion. The anion that is associated with it is also identified in the name. Ammonium salts that have four alkyl groups bonded to nitrogen are called quaternary ammonium salts. ...
... A nitrogen that bears four substituents is positively charged and is named as an ammonium ion. The anion that is associated with it is also identified in the name. Ammonium salts that have four alkyl groups bonded to nitrogen are called quaternary ammonium salts. ...
Alkenes
... 1) Find the longest continuous chain that includes the double bond. 2) Replace the -ane ending of the unbranched alkane having the same number of carbons by -ene. 3) Number the chain in the direction that gives the lowest number to the doubly bonded carbon. ...
... 1) Find the longest continuous chain that includes the double bond. 2) Replace the -ane ending of the unbranched alkane having the same number of carbons by -ene. 3) Number the chain in the direction that gives the lowest number to the doubly bonded carbon. ...
Presentation
... the corresponding hydrocarbons due to (A) intermolecular hydrogen bonding (B) intramolecular hydrogen bonding (C) van der Waal’s forces of attraction (D) dipole – dipole interactions ...
... the corresponding hydrocarbons due to (A) intermolecular hydrogen bonding (B) intramolecular hydrogen bonding (C) van der Waal’s forces of attraction (D) dipole – dipole interactions ...
File
... Boiling points increase. The alcohols are all primary and hydrogen bond, the only difference is in the length of the carbon chain. The longer the carbon chain the more weak intermolecular forces between the compounds (b) ...
... Boiling points increase. The alcohols are all primary and hydrogen bond, the only difference is in the length of the carbon chain. The longer the carbon chain the more weak intermolecular forces between the compounds (b) ...
Chemical Reactions
... them out (or give them to your dog.) The rest of the cookies are okay. How many cookies could you have made (theoretical ...
... them out (or give them to your dog.) The rest of the cookies are okay. How many cookies could you have made (theoretical ...
Name: Period:______ Let`s make some sandwiches! Introduction: If
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.