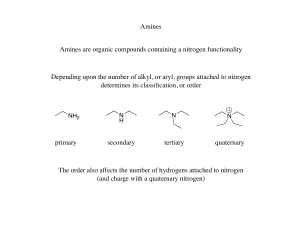
Lectures 4-6
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
... Collins Oxidation (CrO3 • 2pyridine) TL 1969, 336 - CrO3 (anhydrous) + pyridine (anhydrous) ...
4 Expressing and Measuring Chemical Change
... time, most equations can eventually be balanced this way. However, the job can be made much easier if you follow a good plan of attack. Keep the following hints in mind when attempting to balance an equation: • Begin by balancing atoms that appear in only one place on each side of the equation. Atom ...
... time, most equations can eventually be balanced this way. However, the job can be made much easier if you follow a good plan of attack. Keep the following hints in mind when attempting to balance an equation: • Begin by balancing atoms that appear in only one place on each side of the equation. Atom ...
Chapter 19
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
... Amine salts are also used to catalyze a variety of organic reactions that feature two components that are soluble in different liquid phases (e.g. organic and aqueous) ...
Fluorinated Butatrienes - diss.fu-berlin.de
... wurde ein Enin-Isomer entdeckt, das erstaunlicherweise stabiler als sein Butatrien Isomer ist, obwohl es an der Dreifachbindung fluoriert ist. Eben jene Fluorierung an der Dreifachbindung ist eigentlich notwendig um die Energie fluorierter But-1-en-3-ine relativ ...
... wurde ein Enin-Isomer entdeckt, das erstaunlicherweise stabiler als sein Butatrien Isomer ist, obwohl es an der Dreifachbindung fluoriert ist. Eben jene Fluorierung an der Dreifachbindung ist eigentlich notwendig um die Energie fluorierter But-1-en-3-ine relativ ...
4797 Chem Test Ch 21
... MULTIPLE CHOICE On the line at the left of each statement, write the letter of the choice that best completes the statement or answers the question. ...
... MULTIPLE CHOICE On the line at the left of each statement, write the letter of the choice that best completes the statement or answers the question. ...
Chapter 22 and 23 Study Guide
... Be able to define all boldface words from Ch. 20. How many valence electrons does a carbon atom have? How many covalent bonds can each carbon atom form? How is ethanol produced in nature? Be able to write the molecular formula of alkanes, alkenes and alkynes with 1-10 carbon atoms. Example: what is ...
... Be able to define all boldface words from Ch. 20. How many valence electrons does a carbon atom have? How many covalent bonds can each carbon atom form? How is ethanol produced in nature? Be able to write the molecular formula of alkanes, alkenes and alkynes with 1-10 carbon atoms. Example: what is ...
Role of Chemical Reaction Engineering in Sustainable
... fluidized beds, much smaller (compared to packed bed) catalyst particles on support (20 to 150 μm ) are used and these must exhibit outstanding attrition properties. Hence, extensive catalyst development was required to move butane oxidation from fixed to fluidized beds. In the mid 1990s, another i ...
... fluidized beds, much smaller (compared to packed bed) catalyst particles on support (20 to 150 μm ) are used and these must exhibit outstanding attrition properties. Hence, extensive catalyst development was required to move butane oxidation from fixed to fluidized beds. In the mid 1990s, another i ...
10 | Carbon: More Than Just Another Element
... first four of which are CH4 (methane), C2H6 (ethane), C3H8 (propane), and C4H10 (butane) (Figure 10.4). Methane has four hydrogen atoms arranged tetrahedrally around a single carbon atom. Replacing a hydrogen atom in methane by a OCH3 group gives ethane. If an H atom of ethane is replaced by yet ano ...
... first four of which are CH4 (methane), C2H6 (ethane), C3H8 (propane), and C4H10 (butane) (Figure 10.4). Methane has four hydrogen atoms arranged tetrahedrally around a single carbon atom. Replacing a hydrogen atom in methane by a OCH3 group gives ethane. If an H atom of ethane is replaced by yet ano ...
K eq
... No - The paper wads keep flying in both directions even after equilibrium is achieved ...
... No - The paper wads keep flying in both directions even after equilibrium is achieved ...
Unit 8: Reactions
... The mass on the reactants (left) side of the arrow and the mass on the products (right) side of the arrow MUST equal each other as the Law of Conservation of Mass states that mass may not be created or destroyed in any chemical reaction or physical change. Missing Mass examples: 1. Given that 35.0 g ...
... The mass on the reactants (left) side of the arrow and the mass on the products (right) side of the arrow MUST equal each other as the Law of Conservation of Mass states that mass may not be created or destroyed in any chemical reaction or physical change. Missing Mass examples: 1. Given that 35.0 g ...
Review - gbschemphys
... Suppose that a student wishes to solve a problem involving the determination of the mass of product produced if a given amount of moles of reactant was reacted. Which quantities would be essential in order to solve such a problem? Bubble in all that apply - but only those that are essential to this ...
... Suppose that a student wishes to solve a problem involving the determination of the mass of product produced if a given amount of moles of reactant was reacted. Which quantities would be essential in order to solve such a problem? Bubble in all that apply - but only those that are essential to this ...
+ :O
... NOTE: Both of the above types of derivatives have intermolecular attractions similar to those of esters, and so they have boiling points in the same range as esters of comparable size. Both of these types of derivatives are important, powerful donors of their acyl groups and find much use in synthes ...
... NOTE: Both of the above types of derivatives have intermolecular attractions similar to those of esters, and so they have boiling points in the same range as esters of comparable size. Both of these types of derivatives are important, powerful donors of their acyl groups and find much use in synthes ...
Microwave Irradiation for the Facile Synthesis of
... orange-red Os-, and yellow Mn- and W-NP dispersions; and dark-brown to black Mo-, Re-, Ru-, Co-, Rh-, or Ir-NP dispersions (Figure S2 in the Supporting Information) were reproducibly obtained through microwave decomposition at 10 W in 3 min from their metal carbonyl in [BMIm]ACHTUNGRE[BF4]. Complete ...
... orange-red Os-, and yellow Mn- and W-NP dispersions; and dark-brown to black Mo-, Re-, Ru-, Co-, Rh-, or Ir-NP dispersions (Figure S2 in the Supporting Information) were reproducibly obtained through microwave decomposition at 10 W in 3 min from their metal carbonyl in [BMIm]ACHTUNGRE[BF4]. Complete ...
Topic 5 Energetics File
... Born-Haber cycle: Energy cycles for the formation of ionic compounds. If there is little agreement between the theoretical and experimental values, this could indicate a degree of covalent character. Electron affinity: Enthalpy change when an electron is added to an isolated atom in the gaseous stat ...
... Born-Haber cycle: Energy cycles for the formation of ionic compounds. If there is little agreement between the theoretical and experimental values, this could indicate a degree of covalent character. Electron affinity: Enthalpy change when an electron is added to an isolated atom in the gaseous stat ...
Recent advances in homogeneous nickel catalysis
... have been disclosed. Benzylic cross-coupling Another mode of bond activation that has come to prominence, at least in part thanks to nickel catalysis, is the activation of benzylic C–O bonds37. Benzylic ethers, esters, carbonates, carbamates and, in some instances, even free alcohols (via the corre ...
... have been disclosed. Benzylic cross-coupling Another mode of bond activation that has come to prominence, at least in part thanks to nickel catalysis, is the activation of benzylic C–O bonds37. Benzylic ethers, esters, carbonates, carbamates and, in some instances, even free alcohols (via the corre ...
Chapter 9 Reaction Energetics
... * Actually, the term mass-energy should be used instead of energy because mass and energy can be converted into one another by E = mc2. In nuclear reactions, where large amounts of energy are involved, there are substantial changes in mass. However, the mass changes in chemical processes are undetec ...
... * Actually, the term mass-energy should be used instead of energy because mass and energy can be converted into one another by E = mc2. In nuclear reactions, where large amounts of energy are involved, there are substantial changes in mass. However, the mass changes in chemical processes are undetec ...
Chemistry 162 Workbook 10.6
... In order to get the most out of the practice exams, it is imperative to simulate a test-‐like environment when taking these exams. Many students unfortunately suffer from test anxiety ...
... In order to get the most out of the practice exams, it is imperative to simulate a test-‐like environment when taking these exams. Many students unfortunately suffer from test anxiety ...
Alkenes notes
... Heterolytic fission is the breaking of a covalent bond which results in both electrons going to the same atom. This is in contrast to alkanes which can only react with free radicals and undergo homolytic fission. An electrophile is a species which can accept a pair of electrons from a species with a ...
... Heterolytic fission is the breaking of a covalent bond which results in both electrons going to the same atom. This is in contrast to alkanes which can only react with free radicals and undergo homolytic fission. An electrophile is a species which can accept a pair of electrons from a species with a ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.