Oxidation
... alkenes. For example in case of substituted cinnamyl alcohols, an electron-withdrawing (p-nitro) group decreases the reaction rate while electron donating (p-methoxy) increase the rate of reactions. ...
... alkenes. For example in case of substituted cinnamyl alcohols, an electron-withdrawing (p-nitro) group decreases the reaction rate while electron donating (p-methoxy) increase the rate of reactions. ...
New insights into the mechanism of sorbitol transformation
... liquid was detected in the separator, the reactor was pressurized to the required pressure (22–37 bar), and then heated at 3 °C min −1 to the required temperature (200–240 °C). When the required temperature and pressure were achieved, the aqueous solution containing the reactant was co-fed with hydr ...
... liquid was detected in the separator, the reactor was pressurized to the required pressure (22–37 bar), and then heated at 3 °C min −1 to the required temperature (200–240 °C). When the required temperature and pressure were achieved, the aqueous solution containing the reactant was co-fed with hydr ...
中国科学技术大学有机合成讲义-2
... - DMP has found wide utility in the preparation of sensitive, highly functionalized molecules. DMP oxidations are characterized by short reaction times, use of a single equivalent of oxidant, and can be moderated with regard to acidity by the incorporation of additives such as pyridine. DMP and its ...
... - DMP has found wide utility in the preparation of sensitive, highly functionalized molecules. DMP oxidations are characterized by short reaction times, use of a single equivalent of oxidant, and can be moderated with regard to acidity by the incorporation of additives such as pyridine. DMP and its ...
the suzuki-miyaura reaction and boron reagents – mechanism
... (21) Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1971, 93, 1816. (22) Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1972, 94, 4370. (23) Mannig, D.; Nöth, H. Angew. Chemie Int. Ed. English 1985, 24, 878. (24) Crudden, C. M.; Edwards, D. European J. Org. Chem. 2003, 2003, 4695. (25) Carroll, A.-M.; O ...
... (21) Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1971, 93, 1816. (22) Brown, H. C.; Gupta, S. K. J. Am. Chem. Soc. 1972, 94, 4370. (23) Mannig, D.; Nöth, H. Angew. Chemie Int. Ed. English 1985, 24, 878. (24) Crudden, C. M.; Edwards, D. European J. Org. Chem. 2003, 2003, 4695. (25) Carroll, A.-M.; O ...
Amines
... Preparation of Amines t Nucleophilic Substitution Reactions l Alkylation of Ammonia with an alkyl halide è Initial aminium salt is treated with base to give the primary amine è The method is limited because multiple alkylations usually occur ...
... Preparation of Amines t Nucleophilic Substitution Reactions l Alkylation of Ammonia with an alkyl halide è Initial aminium salt is treated with base to give the primary amine è The method is limited because multiple alkylations usually occur ...
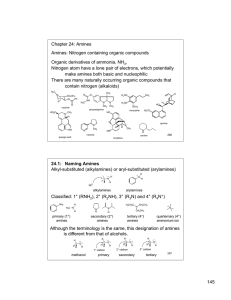
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Synthesis of primary amines from the reaction of alkyl halides or tosylates with “ammonia equivalents” Azide ion is a very strong nucleophile and react with 1° or 2° alkyl halides or tosylates via an SN2 reaction. The resulting azide can be reduced to a 1° amine. H N N N ...
... Synthesis of primary amines from the reaction of alkyl halides or tosylates with “ammonia equivalents” Azide ion is a very strong nucleophile and react with 1° or 2° alkyl halides or tosylates via an SN2 reaction. The resulting azide can be reduced to a 1° amine. H N N N ...
Colorimetric Assay of Alditols in Complex Biological Samples
... periodate consumed. Because the periodate oxidation of alditols is very rapid, errors due to oxidation of other substances present in the material were found to be negligible. Further developments of the chemical determination of mannitol, and other polyalcohols, involved the colorimetric determinat ...
... periodate consumed. Because the periodate oxidation of alditols is very rapid, errors due to oxidation of other substances present in the material were found to be negligible. Further developments of the chemical determination of mannitol, and other polyalcohols, involved the colorimetric determinat ...
Full cell simulation and the evaluation of the buffer system on air
... that the substrates and biomass reach a dynamic balance [12]. In the MFC system, the biofilms will mature regardless of substrate abundance in the reactor [5,18]. Therefore, the thickness and biomass density of the biofilms in MFC reactor are assumed to be unchanged in this steady state model, shown i ...
... that the substrates and biomass reach a dynamic balance [12]. In the MFC system, the biofilms will mature regardless of substrate abundance in the reactor [5,18]. Therefore, the thickness and biomass density of the biofilms in MFC reactor are assumed to be unchanged in this steady state model, shown i ...
chemical kinetics type 1.mdi
... Photochemical reactions. Those reactions which take place in the presence of light are called photochemical reactions. Photosynthesis is an example of photochemical reaction. Photosensitization. The process in which a molecule that absorbs light transfers its extra energy to another molecule which m ...
... Photochemical reactions. Those reactions which take place in the presence of light are called photochemical reactions. Photosynthesis is an example of photochemical reaction. Photosensitization. The process in which a molecule that absorbs light transfers its extra energy to another molecule which m ...
DEPARTMENT OF CHEMISTRY, UNIVERSITY OF JYVÄSKYLÄ
... The understanding of bonding between atoms forms the foundation of chemistry. Although modern quantum theory has helped to clarify the details of the formation of molecules with great accuracy, less is still known about what the molecules can do with their bonds. The reactivity of low-valent main gr ...
... The understanding of bonding between atoms forms the foundation of chemistry. Although modern quantum theory has helped to clarify the details of the formation of molecules with great accuracy, less is still known about what the molecules can do with their bonds. The reactivity of low-valent main gr ...
Anionic rearrangement of 2-benzyloxypyridine derivatives and a synthetic approach to aldingenin B
... TABLE OF CONTENTS List of Tables .......................................................................................................... vii List of Figures ........................................................................................................ viii Abstract .................... ...
... TABLE OF CONTENTS List of Tables .......................................................................................................... vii List of Figures ........................................................................................................ viii Abstract .................... ...
Graphene-Catalyzed Direct Friedel–Crafts Alkylation Reactions
... recovered GO (pH = 3.95 at 0.29 mg mL−1) indicated the slightly acidic nature of the GO carbocatalyst, and no changes in the acidity after the reaction.48 (5) EDXS analysis (energydispersive X-ray spectroscopy) of the GO material before the reaction showed C/O atomic ratio of 1.95, which increased t ...
... recovered GO (pH = 3.95 at 0.29 mg mL−1) indicated the slightly acidic nature of the GO carbocatalyst, and no changes in the acidity after the reaction.48 (5) EDXS analysis (energydispersive X-ray spectroscopy) of the GO material before the reaction showed C/O atomic ratio of 1.95, which increased t ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.