* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Full cell simulation and the evaluation of the buffer system on air
Chemical equilibrium wikipedia , lookup
Asymmetric induction wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Analytical chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Fluorescence correlation spectroscopy wikipedia , lookup
Rate equation wikipedia , lookup
Theory of solar cells wikipedia , lookup
History of electrochemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
IsaKidd refining technology wikipedia , lookup
Scanning electrochemical microscopy wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Cathodic protection wikipedia , lookup
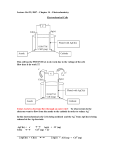
Journal of Power Sources 347 (2017) 159e169 Contents lists available at ScienceDirect Journal of Power Sources journal homepage: www.elsevier.com/locate/jpowsour Full cell simulation and the evaluation of the buffer system on air-cathode microbial fuel cell Shiqi Ou a, *, Hiroyuki Kashima b, Douglas S. Aaron c, John M. Regan b, Matthew M. Mench c a b c National Transportation Research Center, Oak Ridge National Laboratory, Knoxville, TN, 37932, USA Department of Civil & Environmental Engineering, The Pennsylvania State University, University Park, PA, 16801, USA Department of Mechanical, Aerospace and Biomedical Engineering, The University of Tennessee, Knoxville, TN, 37996, USA h i g h l i g h t s The full cell model provides a macro/micro perspective of the MFC interrelation. Study of the effects of mass transport drivers (diffusion and electric migration). Analysis of the Hþ/OH- transport and pH change in the whole cell system. Overall impacts of various buffers on the whole reactor are compared. Quantitative delineation of overpotentials at both anode and cathode in MFC. a r t i c l e i n f o a b s t r a c t Article history: Received 13 October 2016 Received in revised form 19 January 2017 Accepted 10 February 2017 This paper presents a computational model of a single chamber, air-cathode MFC. The model considers losses due to mass transport, as well as biological and electrochemical reactions, in both the anode and cathode half-cells. Computational fluid dynamics and Monod-Nernst analysis are incorporated into the reactions for the anode biofilm and cathode Pt catalyst and biofilm. The integrated model provides a macro-perspective of the interrelation between the anode and cathode during power production, while incorporating microscale contributions of mass transport within the anode and cathode layers. Model considerations include the effects of pH (Hþ/OH transport) and electric field-driven migration on concentration overpotential, effects of various buffers and various amounts of buffer on the pH in the whole reactor, and overall impacts on the power output of the MFC. The simulation results fit the experimental polarization and power density curves well. Further, this model provides insight regarding mass transport at varying current density regimes and quantitative delineation of overpotentials at the anode and cathode. Overall, this comprehensive simulation is designed to accurately predict MFC performance based on fundamental fluid and kinetic relations and guide optimization of the MFC system. © 2017 Elsevier B.V. All rights reserved. Keywords: Microbial fuel cell Computational simulation Mass transport Buffer system pH Concentration overpotential 1. Introduction Microbial fuel cells (MFCs) have been the subject of years of research; despite much effort, the performance of MFCs has not improved to the point that they are economically viable as power sources. Part of this slow development is due to the need for greater * Corresponding author. National Transportation Research Center, Energy and Transportation Sciences Division, Oak Ridge National Laboratory, Knoxville, TN 37932, USA. E-mail address: [email protected] (S. Ou). http://dx.doi.org/10.1016/j.jpowsour.2017.02.031 0378-7753/© 2017 Elsevier B.V. All rights reserved. fundamental understanding of how these devices convert wastewater streams into electricity e bioelectricity generation from biomass by using organic substrates [1]. In the MFC system, the microorganisms on the anode generate electrons by consuming organic matter; electrons then flow to the anode via self-produced mediators or nanowires [2]. The electrons are conducted through an outer circuit to the cathode, permitting reduction reactions to occur, typically on a cathodic catalyst. In certain MFC reactors (e.g., two-chamber design), a membrane (e.g., cationic, anionic, or ultrafiltration membrane) is placed between the anode and cathode to prevent electrical shortage and minimize oxygen infiltration to the anode-respiring bacteria (ARB). However, this design increases 160 S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 the internal resistance and reduces the power output [1], increasing the system cost [3]. A single chamber air-cathode reactor uses air passively supplied to the cathode and avoids the cost of a membrane; in this design, the cathode environment shares bulk liquid with the anode electrode [3]. Historically, most MFC development has been experimentbased. Recent efforts at simulation allow multiple controlling phenomena to be considered, which can guide system optimization [4]. Building off the MFC models have been refined and expanded to become useful tools for MFC behavior analysis. The initial focus for most MFC models was regional analysis of the anode and electron generation: Picioreanu et al. described transient anodic biofilm growth model from 1D to 3D [5]; Strycharz-Glaven et al. presented a mathematical model focused on anode biofilm conductivity [6]. Although MFC model development has progressed, few models focus on cathode performance, and even fewer couple the anode and cathode into a comprehensive, whole-cell model [7]. In the single chamber MFC reactor, protons (Hþ) are produced during microbial respiration while electrons are transported to the anode. The protons accumulate near the anode and then diffuse via concentration gradient through the bulk liquid to the cathode. The anode pH is typically below 7 as protons accumulate and develop a concentration gradient; experiments have shown that acidic pH results in a significant decrease in voltage efficiency, current density, and resultant power output by negatively affecting ARB metabolism via Le Chatlier's principle [8,9]; this effect is illustrated by the half reaction for acetate oxidation in the anode, shown in Eqn. (1). contributes to inhibited power output. In a single-chamber MFC reactor, cathode pH spans a range from 7.0 to 13.0, according to multiple studies [15,17]. Considering pH effects in both the anode and cathode regions, pH regulation and Hþ/OH mass transport mechanisms are important topics for MFC development. In this modeling work, electric migration and the effects of pH on concentration-driven diffusion were included in mass transport calculations for varying buffer systems. 2. Methods 2.1. Experiments and set up A single-chamber MFC that has been used in earlier work [18] was employed in the series of buffer studies described here. There was no convection present in the system because no stirring or flow was implemented. Anode materials consisted of carbon paper, as indicated in the discussion. Effluent from a mature MFC reactor was used as inoculum, and enriched in batch mode with a growth medium including 50 mM phosphate buffer solution (PBS) and 1.0 g/L sodium acetate as the electron donor. Polarization analysis was conducted by varying external resistance; data points were collected with 20-min time steps while measuring cell voltage and both electrode potentials against an Ag/AgCl reference electrode [19]. Since cell voltage was measured for varying resistors (from 10 M-ohm down to 130 U) connecting the anode and cathode, current was calculated using Ohm's law. 2.2. Model formation 1 3 1 1 þ CH COO þ H2 O/ CO2 þ HCO 3 þH þe 8 3 8 8 8 (1) Electrochemically, lower pH also contributes to increased concentration overpotential for substrate oxidation [9], increasing potential loss for the whole MFC. Because of the numerous impacts of pH, pH regulation is an important consideration for improvement of MFC performance. Several works have detected, via multiple experimental techniques, that the anode biofilm pH is normally in the range of 5e7 [4,10,11]. As a result of these insights, buffer systems have been introduced to stabilize pH, supporting biological growth and decreased anode concentration overpotential [9]. In addition to the kinetic losses associated with microbial respiration, mass transport losses also contribute to cell overvoltage. Such losses are the result of concentration gradient-driven diffusion and migration flux in the MFC when no bulk liquid flow occurs. Convection also affects performance, but is often ignored in experimental single-chamber MFCs. Diffusion is proportional to a concentration gradient while migration is proportional to the bulk liquid electric field and ionic concentration. Prior work has indicated that migration is minor compared to diffusion flux in single chamber MFCs [6,9]. Thus, most MFC modeling efforts only consider diffusion as a mass transport mechanism [5,12-14]. At the cathode, it is increasingly accepted that high pH is due to the generation of OH instead of the consumption of Hþ [15e17]. This reaction, an alternative to typical oxygen reduction to water as in a hydrogen fuel cell, is shown in Eqn. (2). 1 1 O þ e þ H2 O/OH 4 2 2 (2) The bulk liquid separating the anode and cathode thus exhibits a pH gradient from acidic to basic [15]. Accumulation of OH at the cathode affects MFC performance in a manner analogous to Hþ accumulation at the anode: higher pH decreases biomass metabolism efficiency, increases cathode concentration loss, and The steady-state nature of this model assumes constant biomass due to equal detachment and attachment rates in the biofilms and that the substrates and biomass reach a dynamic balance [12]. In the MFC system, the biofilms will mature regardless of substrate abundance in the reactor [5,18]. Therefore, the thickness and biomass density of the biofilms in MFC reactor are assumed to be unchanged in this steady state model, shown in Table 1. The model discussed herein is based on a single chamber air-cathode MFC, an exploded-view schematic of which is shown in Fig. 1 a). The bulk liquid fills a cylindrical space separating the anode and cathode. In the model reactor, the anode electrode is carbon paper instead of the better-performing carbon brush [1]. The carbon paper design simplifies model formulation and optimizes simulation speed, but results in poorer MFC power output. ARB adhere to the carbon paper, forming a relatively thin anodic biofilm. The layered cathode was arranged from the interior to the outside in contact with air as: Pt/C catalyst, hydrophilic carbon cloth, and hydrophobic PTFE. The structural properties of the MFC are included in Table 1, along with other model parameters. Similar to the anodic biofilm, the aerobic cathode microorganisms form a relatively thick biofilm on the Pt/ Carbon catalyst layer. 2.2.1. Mass balance for substrate transport Mass transport relations are formulated for all domains in the MFC, separated based on the physical properties of the materials in each layer. From cathode to anode, they include the PTFE layer, carbon cloth, Pt/C catalyst layer, cathodic biofilm, bulk liquid, anodic biofilm and carbon paper; this structure is shown in Fig. 1b). along with initial and boundary conditions for pH and acetate concentration. Most layers were assumed to be fully saturated with growth medium; however, the cathode carbon cloth was studied by Ou et al. [18] and found to possibly contain a small, ca. 3% by volume, quantity of gas. Additionally, the PTFE layers were assumed to have no liquid present due to their strong hydrophobicity. In the full cell model, the PTFE layer was neglected to simplify calculations S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 161 Table 1 Model parameters for full cell model. Name Description Values Unit cAc,0 cO2,g cO2,ref DAc,liq DCO3,liq DHCO3,liq DH2CO3,liq DHPO4,liq DH2PO4,liq DH3PO3,liq DNH3,liq DNH4,liq DOH,liq DO2-N2 DO2,liq E0C F HO2 KAcH KO2 KO2A KO2H Lbio Lbl Lbdl Lcc Lcl Lpdl pH0 P0 qmax.AcH qmax.Acsus qmax.AcA R Scathode T Xabio Xcbio Xsus εbio εcc εcl εpdl Initial Concentration, sodium acetatea Boundary concentration, gaseous oxygena Reference concentration in reactor, oxygen Diffusion coefficient in liquid, acetateb Diffusion coefficient in liquid, CO2-c 3 Diffusion coefficient in liquid, HCO2-c 3 Diffusion coefficient in liquid, H2COc3 Diffusion coefficient in liquid, HPO2-c 4 Diffusion coefficient in liquid, H2POc 4 Diffusion coefficient in liquid, H3POc4 c Diffusion coefficient in liquid, NH3 Diffusion coefficient in liquid, NHþc 4 Diffusion coefficient in liquid, hydroxidec MS diffusivity, O2eN2 componentb Diffusion coefficient in liquid, oxygenb Cathode equilibrium voltageb (v.s. SHE) Faraday constantb Henry constant, oxygene Half-max-rate acetate concentration, heterotrophic biomass Half-max-rate oxygen concentration in cathode Half-max-rate oxygen concentration, autotrophic biomass Half-max-rate oxygen concentration, heterotrophic biomass Length, cathode biofilm Length, bulk liquidb Length, boundary diffusion layerb Length, carbon clothb Length, Pt/C catalyst layerb Length, PTFE diffusion layerb pH in bulk liquid Gas pressureb Maximum specific rate of acetate utilization, heterotrophic biomass Maximum specific rate of acetate utilization, suspended biomass Maximum specific rate of oxygen utilization, autotrophic biomass Gas constant Cathode cross sectional area in MFCb Temperature Biomass concentration in anodic biofilm Biomass concentration in cathodic biofilm Suspended biomass concentration in liquid Porosity, biofilm Porosity, carbon clothb Porosity, Pt/C catalyst layerb Porosity, PTFE diffusion layers Cathode half-max overpotential Density, bulk liquidb Density, biomass Mass fraction of HAB in biofilm Conductivity, anode biofilm Conductivity, bulk liquidb Conductivity, Pt/C catalyst layer Conductivity, carbon cloth Conductivity, Pt/C catalyst layer 800 237.66 3.79 1.21 109 8.00 1010 1.09 109 1.09 109 7.60 1010 8.80 1010 1.00 109 1.64 109 1.97 109 4.59 109 2.30 105 2.10 109 552 96485 7.79 104 150 0.128 1.28 1.28 1.0 39.0 0.5 0.18 0.032 0.032 7.08 1.01 105 1.00 104 1.00 104 2.00 105 8.314 7.07 104 303.15 24.0 25.0 0.05 0.95 0.75 0.30 0.10 240.028 1.05 1.54 0.85 0.02 0.755 0.05 1.00 105 1.00 103 mg/L mg/L mg/L m2/s m2/s m2/s m2/s m2/s m2/s m2/s m2/s m2/s m2/s m2/s m2/s mV C/mol J/mol mg/L mg/L mg/L mg/L mm mm mm mm mm mm \ Pa (mg Ac)/(mg HAB s) (mg Ac)/(mg SUS s) (mg O2)/(mg AAB s) J/mol K m2 K g/L g/L g/L \ \ \ \ mV g/cm3 g/cm3 \ S/m S/m S/m S/m S/m hK rbl rbio s sabio sbl scbio scc scl e Values for other model parameters were assumed based on common practical experience. a is from experimental data. b is from Ref. [28]. c is from Ref. [29]. d is from Ref. [30]. e is from Ref. [23]. f is from Ref. [31]. and because gas transport in a porous medium is well-known. The amount of dissolved oxygen was calculated according to Henry's law in equilibrium with air. To improve computational accuracy, two boundary diffusion layers were added into the model. These boundary diffusion layers serve as transition regions between the electrodes and bulk liquid [20]. The NernstePlanck equation describes electric field-affected mass transport for each compound and is shown in Eqn. (3). vci ¼ V Fdiff þ Fmig þ ri vt (3) where Fdiff is the diffusion flux: Fdiff ¼ Di vci vx and Fmig is the electric migration flux [9]: (4) 162 S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 Fig. 1. a) Structure of the single chamber air-cathode MFC reactor; b) Schematic diagram for the domains and initial conditions addressed in this model. Fmig ¼ zi F vV D RT i vx (5) In these relationships, i is for each species, ci is concentration (mg/L), Di is effective diffusion coefficient (m2/s), zi is ionic charge (C), V is the local electric potential (mV), and ri is the reaction rate (mg/L s). The reaction rate is characterized by the bioelectrochemical reaction in the anode and cathode biofilms, as well as Pt-catalyzed oxygen reduction in the cathode. It is assumed that ARB respiration is the only operationally-relevant reaction in the anode biofilm, where the ARB transfer electrons from acetate to the anode. Electronic conduction in the anode causes local potential gradients over the anodic biofilm. In addition to the effects of local electronic potential, acetate concentration affects ARB metabolism. The ARB reaction rate is thus related to the local overpotential and local electron source (acetate); the Monod-Nernst equation includes these considerations for ARB metabolism [14]. Eqn. (6) shows this relationship and has been validated by kinetic experiments [21]. cAc rAc;ARB ¼ qAc;ARBmax XARB cAc þ KAc 1 0 1 A @ Fh F 1 þ exp RT act;A þ RThK;A (6) where rAc,ARB is ARB acetate reaction rate (mg/L s), qAc,ARBmax is ARB maximum specific rate of acetate consumption (mg Ac/mg ARB s), XARB is ARB concentration (mg/L), cAc is local acetate concentration (mg/L), hact,A is anodic activation overpotential (V), hK,A is halfmaximum activation overpotential (V) [14], F is the Faraday constant (C/mol), T is temperature (K), and R is the ideal gas constant (J/ mol K). Oxygen reduction occurs at both the Pt/C catalyst layer and in the cathodic biofilm. In the catalyst layer, oxygen is the electron acceptor and OH is generated, as shown in Eqn. (2). While the cathode biofilm is comprised of a consortium of microbes, this study simplifies the cathodic biofilm microbial species into two groups based on their electron sources: autotrophic aerobic biomass (AAB), and heterotrophic aerobic biomass (HAB) [18]. Electrons from the anode are consumed in both the Pt/C layer and S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 in the biofilm [19]. The porous cathode catalyst structure is the same as in conventional PEM fuel cells; the reaction rate calculation in the catalyst layer is described by a simplified Butler-Volmer expression in Eqn. (7) [22]. " # 1 c bF ic;0 O2 MO2 dcl rO2 ;cl ¼ exp h 4F RT act;C cO2 (7) where rO2,cl is oxygen reaction rate in the catalyst layer (mg/L s), ic,0 is the cathode exchange current density (A/m2), c*O2 is the saturation concentration of oxygen (g/L), b is the symmetry factor of the reaction, MO2 is oxygen molecular mass (mg/mol), and dcl is the catalyst specific area (m2/m3). In the cathode biofilm, the MonodNernst equation for reaction rates is used. Eqn. (8) is the AAB reaction rate, and Eqn. (9) is the HAB reaction rate in the cathode biofilm [18].where rO2,AAB is AAB oxygen reaction rate (mg/L s), rO2 ;AAB ¼ qO2 ; AABmax XAAB 163 anode voltage, E0A, is 500.46 mV. The experimental cell voltage is calculated based on the attached external resistance and the measured current as shown in Eqn. (14). The model has been constructed to use external resistance as the defining input, with all other parameters responding appropriately. Vcell ¼ IRext (14) The biofilms in both electrodes are assumed to be electronically conductive, described by an electron balance and Ohm's law [14], shown in Eqn. (15). This relationship is also used for the metallic catalyst in the cathode. 0¼s v2 hact þ Fgr vx2 (15) where s is conductivity (S/m); hact is local activation overpotential cO2 1 F KO2 ;AAB þ cO2 1 þ exp F h RT act;C þ RThK;C (8) cO2 cAc 1 F KO2 ;HAB þ cO2 KAc;HAB þ cAc 1 þ exp F h RT act;C þ RThK;C (9) rAc;HAB ¼ qAc; HABmax XHAB qO2,AABmax is maximum specific rate of oxygen consumption (mg O2/mg AAB s) for AAB, XAAB is the AAB concentration (mg/L), KO2,AAB is the half-max-rate oxygen concentration (mg/L), hact is the cathodic activation overpotential (V), hK,C is the cathodic half-max overpotential (V), rAc,HAB is HAB acetate reaction rate (mg/L s), qAc,HABmax is maximum specific rate of acetate consumption (mg Ac/mg HAB s) for HAB, XHAB is HAB concentration (mg/L). 2.2.2. Current generation Generally, overpotential analysis in MFCs is similar to that in PEMFCs [23]. The overpotential is divided into three sources: activation overpotential hact, ohmic overpotential hohm, and concentration overpotential hcon; all are relevant in both the cathode and anode [23]. The total overpotential htotal is shown in Eqn. (10). The overpotential balance is shown in Eqn. (11). htotal ¼ hact þ hcon þ hohm (10) Vcell ¼ E0C hC;act hC;con E0A þ hA;act þ hA;con jhohm j (11) It is assumed that the internal resistance is comprised of the anodic resistance RA, cathodic resistance RC, and electrolyte resistance in bulk liquid RBL, as shown in Eqn. (12) and Eqn. (13). jhohm j ¼ hA;ohm þ hC;ohm þ hBL;ohm (12) jhohm j ¼ IRA þ IRC þ IRBL (13) The open circuit voltages (v.s. Ag/AgCl) for the anode and cathode in this model have been measured experimentally: the equilibrium cathode voltage, E0c , is 273.3 mV while the equilibrium (V); g is electron equivalence (mol e/mol); r is a particular substrate reaction rate (mol/L s): acetate consumption for ARB in the anode or HAB in the cathode, and oxygen consumption for AAB in cathodic biofilm, or ORR in cathodic catalyst layer. This steady state equation is calculated based on two boundary conditions [14], which are shown in Eqn. (16) and Eqn. (17), respectively. hact jx¼0 ¼ hele;act (16) vhact ¼0 vx x¼L (17) where hele,act is local activation overpotential at the electrode. Eqn. (16) is the condition at the boundaries between conductive electrode materials (anode carbon paper and cathode carbon cloth) and current collectors shown in Fig. 1 a). Eqn. (17) is the condition at the boundaries between the boundary diffusion layers and biofilms. In Eqn. (17), L is the total length of the anode biofilm and carbon paper in the anode; for the cathode, the relevant L is the length of the cathodic biofilm, Pt/C layer, and carbon cloth, shown in Fig. 1 b). 2.2.3. Buffer system This work includes the effects of several common buffers, including: NH4Cl, NaHCO3, and PBS (phosphate buffered system). The relationship between pH and ARB metabolism or ARB growth is difficult to experimentally analyze [13], thus this study only considered the effects of buffers on overpotentials that respond to pH. Eqn. (18) shows the concentration overpotential (hcon,pH) related to pH values. hcon;pH cHþ =OH RT ln 0 ¼ nF cHþ =OH ! (18) where the anode concentration overpotential is dependent upon 164 S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 Table 2 Buffer chemical reactions in simulation. Buffer name Equation pKa H2O NH4Cl NaHCO3 H2O 4 OH þ Hþ þ NHþ 4 4 NH3 þ H þ H2CO3 4 HCO 3 þ H þ 2HCO 3 4 CO3 þ H þ H3PO4 4 H2PO 4 þ H þ 2H2PO 4 4 HPO4 þ H þ 3HPO24 4 PO4 þ H 14 9.25 [32] 6.37 [33] 10.3 [33] 2.12 [34] 7.21 [34] 12.32 [34] PBS Hþ concentration, while the cathode depends on OH concentration. Local pH in the bulk liquid is assumed to be influenced by proton transport from the anode and hydroxide transport from the cathode; cHþ/OH- is local Hþ or OH concentration and c0Hþ/OH- is initial Hþ or OH concentration in the bulk liquid. The initial pH is set to be 7.08, based on experimental measurement. Table 2 lists the buffer chemical dissociation equations adopted in the model. An algorithm has been established for calculating the buffer equilibria and other reactions in all MFC domains. The algorithm with related equations is shown as a flow diagram in Fig. 2. Via Eqns. (6)e(9), rAc, rO2 and rH are obtained in every domain. Eqn. (3) gives the mass transport calculations for each species; concentrations (including pH) are updated for each iteration until the residuals between adjacent iterations are smaller than the tolerances. These reactions and mass transport affect pH, thus chemical and electrochemical reactions must be rebalanced. The buffers obey typical dissociation behavior, shown in Eqns. (19) and (20). Fig. 3. Influence of electric migration inclusion on simulation of MFC power production. ðc ÞðcHþ Þ Ka ¼ B cBH (19) BH4B þ Hþ (20) Ka is the dissociation constant, while cBH and cB- represent the concentrations of undissociated and deprotonated buffer in the equilibrium, respectively. This model assumes that the dissociation Fig. 2. Flow diagram of the algorithm for buffer chemical reactions and pH in MFC system modeling. Fig. 4. Polarization and power density curves for an experimental MFC with 50 mM buffer and simulations of unbuffered, 50 mM buffered, and an ideal scenario in which pH effects are neglected. S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 reaction rates (Eqn. (20)) are much faster than the timescale of mass transport [13]. Thus, updated local concentrations of B and BH are defined by Eqn. (19). Stepping through the algorithm, results from mass transport calculations update in each control volume, a new balance is established, and new pH values are obtained based on Eqn. (19), as shown in Fig. 2. The residual of the pH values in adjacent iterations is calculated and compared to a tolerance to decide the next computational step. Picioreanu et al. [13] also presented a method for simulating the pH of a buffer in a MFC half-cell, which included dissociation rate. This work defined the dissociation equilibrium reaction rate as shown in Eqn. (21): c c þ ra;BH ¼ kBH cBH B H Ka (21) where, kBH is the rate constant for the dissociation equilibrium (s1). In this study, both the proposed buffer algorithm shown above and the Picioreanu et al. algorithm (with kBH assumed to be 1010 s1) were compared in the full cell model for calculating the pH values as well as the buffer concentrations. Modeling results for these two algorithms were compared and the maximum disagreement between these two algorithms was less than 0.8%. 165 3. Results and discussion Three cases were discussed in the study: 1) Baseline experimental results simulation with concentration overpotential impact analysis; 2) MFC performance comparison for multiple buffer solutions; 3) comparison of varying buffer concentrations. In the steady state model, the external resistance was decreased from 4000 U to 50 U. The electrochemical results (e.g., polarization curves, power density, overpotentials), species distributions (e.g., oxygen, acetate, buffer chemicals), and pH profiles in the full cell were simulated and evaluated. 3.1. Numerical evaluation for electric migration in the mass transport Mass transport in the microbial fuel cell can be influenced by convection, diffusion and electric migration. Convection was neglected in the model because there was no stirring in or flow through the reactor. The relative influences of diffusion and electric migration were compared by assessing two mass transport scenarios against relevant experimental data: once with only diffusion and once with both drivers of transport. In Eqn. (22), the two Fig. 5. a) pH distribution across the MFC at varying current densities for the 50 mM buffer simulation; b) Maximum cathode pH and minimum anode pH responses to current density for unbuffered and 50 mM buffered MFCs. 166 S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 scenarios were with or without the second diffusion term in parentheses [19]: vci v ¼ vx vt Deff i vci zi F eff vE Di þ ri vx vx RT (22) where i is for each species, ci is concentration, Deff i is the effective diffusion coefficient, zi is charge of any ion, E is local electric potential, and ri is reaction rate. We note that, in this assessment, the model was first compared to the experimental data and obtained very good fit, as shown in Fig. 3. After obtaining all model parameters for diffusion-only mass transport, the model was modified to include electric migration and refit to the experimental data while retaining the values obtained in the diffusion-only case; this analysis allowed the illustration of the impact of electric migration by slightly overestimating MFC output. As shown in Fig. 3, inclusion of electric migration flux in mass transport for all three major regions of the MFC resulted in over prediction of the maximum power output by 7.4% compared to the scenario in which electric migration is neglected, as well as the experimental results. Because the impact of migration is small under these conditions, it is neglected in some modeling work [9,24,25]. For the comparison of multiple buffer concentrations in Section 3.4, electric migration was included in the model because buffer concentration affects the conductivity of the bulk liquid. However, the purpose of this study was to compare the performances between different buffer solutions. Thus, the experimental results serve as a reference for calibration and comparison in the study. 3.2. Simulation of buffer impact As seen in Fig. 4, the model fit the experimental results very well when a 50 mM buffer was included and electric migration was neglected. The simulated buffer consisted of 50 mM phosphate buffer (17.77 mM NaH2PO4/H2O and 32.23 mM Na2HPO4) along with 1.74 mM KCl and 5.79 mM NH4Cl. The next comparison removed the influence of a buffer and the effects of pH from all reactions. These two scenarios represent a relatively poor case (uncontrolled pH) and an ideal case (where Hþ and OH concentrations do not affect concentration overpotential), respectively. Aside from buffer behavior, all parameters used in this section are as described in Table 1. These three scenarios are shown, along with the experimental data, in Fig. 4. Shown in Fig. 4, simulated MFC power output in the absence of any buffer (but with pH effects on concentration overpotential) averaged ~9.80% less than the 50 mM buffer case. The lower output from the unbuffered simulation is primarily due to increased overpotential on the cathode; the anode overpotential also increased slightly compared with the buffered scenario. When pH effects on concentration overpotential were neglected (the ideal case), the MFC produced ~21.9% higher power than the 50 mM buffer scenario. The overpotentials in both the anode and cathode sides were obviously smaller in this ideal simulation. The pH distribution across the entire cell was also analyzed. Fig. 5 a) shows the calculated distribution of pH across the MFC for varying current densities. The cathodic pH is defined by the production of OH, while the anodic pH is due to Hþ production. The bulk liquid initial pH was set to an experimentally-measured 7.08; Fig. 5 a) shows that pH < 7.08 over most of the bulk liquid while Fig. 6. a) Polarization and power density curves; and b) pH profiles for different buffer solutions. S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 167 pH > 7.08 is true for the entire cathode. This asymmetry arises because the Hþ diffusion coefficient is much larger than that of OH, facilitating faster proton diffusion in the bulk liquid, and a resultant slightly acidic bulk liquid. Fig. 5 b) shows the maximum pH in the cathode and minimum pH in the anode over a range of current density for both unbuffered and 50 mM buffered scenarios. Since the “Ideal” simulation ignored pH influence, this profile is not included in Fig. 5 b). It is apparent that the 50 mM buffer moderates the changes in pH for both halves of the MFC, especially in the anode. According to Eqn. (18), Hþ/OH concentration affects MFC mass transport overpotential. Since the buffer solution moderately changes in pH, the concentration overpotential is less severe, leading to increased power production. However, it is apparent from the simulation results that the 50 mM buffer solution has limited capacity to accommodate changes in pH. If the “Ideal” simulation is the best-case scenario in which the pH influence by the concentration overpotential is completely eliminated, 50 mM buffer mitigates approximately 31% of the pH influence on MFC performance. Because buffer compounds cannot instantly move throughout the electrodes, it is expected that pH effects on concentration overpotential cannot be completely circumvented. Any activity in the anode or cathode will cause pH to deviate from ~7, even if the bulk liquid pH may be close to neutral. 3.3. Comparison of multiple buffer solutions To assess the effect of buffer type, with attendant differences in buffer mass transport, two other buffers were considered. In this section, 50 mM PBS, 50 mM NaHCO3, and 50 mM NH4Cl were considered in the model with dissociation behavior as outlined in Table 2. In Fig. 6 a), simulation results for polarization and power density curves are presented. The type of buffer did not greatly affect MFC output in the steady-state model. The 50 mM PBS yielded the best performance, though the anode was barely affected, indicating that the cathode responded relatively sensitively to the buffer type. Overall, the peak power for the PBS simulation was only 5% greater than the peak power output for the NH4Cl system. Fig. 6 b) shows trends in maximum cathode pH and minimum anode pH for increasing current density for the three buffer systems. It is apparent that any buffer solution was able to mitigate pH changes for both the anode and cathode compared to a MFC with no buffer. Of the buffers considered, 50 mM PBS buffer was most effective in the anode. pH regulation capability of the PBS buffer was less superior in the cathode when the current density neared the maximum. This superior performance is likely due to the properties shown in Table 2: there are three equilibrium reactions for phosphate species, allowing more Hþ to be buffered. However, phosphate has a smaller diffusion coefficient, limiting PBS mass transport; this effect becomes more pronounced at high current in the thick electrode, leading to the tighter spread of pH between buffers at high current. In the cathode layers, porosity exacerbates buffer mass transport limitations by limiting Hþ transport from the anode to participate in pH neutralization. In this model, the diffusion coefficient of PO34 is an order of magnitude smaller than that for both NHþ 4 and HCO3 , shown in Table 2. The model in this study can yield microscale insights regarding concentration potential losses. Fig. 7 a) shows the total concentration overpotentials in the whole cell MFC (including both cathode and anode) for multiple buffer liquids. As expected, concentration overpotential increases with current density, but this increase in overpotential is mitigated in the presence of any buffer. It appears as though, under these conditions, each of the buffer systems is similarly effective, with 50 mM PBS slightly Fig. 7. a) Concentration overpotentials at varying external resistance for all buffer systems considered; b) Cathode concentration overpotential; c) Anode concentration overpotential. outperforming the others. However, concentration overpotential is typically a relatively minor contributor to total MFC overpotential; this observation is evident here, as well, since the buffers show little variability between themselves [19]. Having compared the total MFC concentration overpotential, the model also allows investigation of concentration overpotential in the anode and cathode. Fig. 7b) and c) respectively show the anode and cathode overpotentials with the four buffer scenarios. It is confirmed in Fig. 7 b) that PBS most effectively reduces concentration overpotential losses in the anode side, followed by NaHCO3 and then NH4Cl. This same trend occurs for the total concentration overpotential losses shown in Fig. 7 a). In Fig. 7 c), the PBS buffer actually performs worst at small external resistance (at high current density), though the difference is small. This agrees with the PBS simulation results in Fig. 6. Since phosphate is a relatively large molecule, with a correspondingly smaller diffusion coefficient compared with HCO 3 and NH3, its mass transport through electrode layers is slower. This is most evident in the cathode at higher current density. 168 S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 Fig. 8. Polarization curves and pH for different PBS concentration solutions. 3.4. Effect of PBS concentration 4. Conclusions The bulk liquid conductivity was kept constant in previous simulations since the buffer concentrations were identical. However, bulk liquid conductivity variations strongly influence MFC power production by affecting ohmic overpotential. To assess this effect, changes in the phosphate buffer concentration were simulated for the full cell system. The polarization and power density curves for 50 mM, 100 mM and 200 mM PBS are shown in Fig. 8. The conductivity of bulk liquid with 50 mM PBS has been measured at 5.9 mS/cm [26], with 100 mM PBS it is 10.2 mS/cm [26], and with 200 mM PBS it is 22.0 mS/cm [27]. As expected for the increase in bulk liquid conductivity, greater PBS concentration yielded decreased internal resistance and a more neutral pH environment, thus enabling greater power output. While PBS yields the best performance, it is also the most expensive buffer considered here; this provides an optimization point to balance increased system cost against improved power density. It is evident that the pH change from 50 mM to 100 mM PBS is larger than the pH changes from the 100 mM and 200 mM curve shown in Fig. 8 b). Due to the dissociation equilibria of PBS, its pH buffering performance does not linearly improve with the amount of PBS present. The buffer liquid has two major roles in the MFC system: 1. create a pH-friendly environment for biomass growth (biological influence); 2. balance ionic concentration/pH for mass transport and electrochemical reactions (electrochemical influence). The simulation results shown in Fig. 8 reflect the impacts of the electrochemical influence in this analysis, therefore the differences of the power outputs shown in Fig. 8 a) are not as great as expected from experimental results elsewhere [26], however the pH curves showed large differences for different amounts of PBS buffers in Fig. 8b). Mass transport of Hþ/OH strongly influences MFC power output due to impacts on microbial metabolism. A model for a single chamber MFC system, including the anode, bulk liquid, and cathode, was used here to analyze mass transport drivers (diffusion and electric migration), as well as biological and electrochemical reactions in the whole system. A numerical method was devised for combinations of anode and cathode subsystems, two relatively independent components of the model, and then used for a steady state simulation at each external resistance. Electric migration was found to have a minor influence on current generation in the single chamber MFC. The effects of three buffer solutions (PBS, NaHCO3, NH4Cl) were simulated in the steady state full cell model, with the PBS buffer solution proving most capable of pH neutralization. However, the large diffusion coefficient for PBS inhibited its performance at high current density. When multiple concentrations of PBS were compared, maximum power output occurred for the highest PBS concentration. This model quantitatively described the mass transport and electrochemical reactions by mathematical algorithm optimization, and provided scenario analysis for full cell MFC system with various buffer solutions, which are difficult to be accomplished experimentally. However, this work captured impacts of pH on multiple overpotentials, while neglecting pH influence on suspended biomass growth in the bulk liquid, which could introduce minor inaccuracy in power outputs. Therefore, more work is needed to quantitatively consider the pH effect on the biomass growth in both anodic and cathodic sides. Acknowledgments This research was supported by the US Army Research Office, contract number: W911NF-11-1-0531. S. Ou et al. / Journal of Power Sources 347 (2017) 159e169 References [1] B.E. Logan, Microbial Fuel Cells, John Wiley & Sons, Inc., Hoboken, New Jersey, 2008. €der, J. Keller, S. Freguia, [2] B.E. Logan, B. Hamelers, R. Rozendal, U. Schro P. Aelterman, W. Verstraete, K. Rabaey, Environ. Sci. Technol. 40 (2006) 5181e5192. [3] Z. He, Y. Huang, A.K. Manohar, F. Mansfeld, Bioelectrochemistry 74 (2008) 78e82. ~ oz, J. Mas, Influence of pH and [4] A. Torrents, N. Godino, F.J. Del Campo, F.X. Mun carbonate buffering on the performance of a lactate microbial fuel cell, in: COMSOL Conf., COMSOL, Paris, 2010. https://www.comsol.com/paper/ influence-of-ph-and-carbonate-buffering-on-the-performance-of-a-lactatemicrobia-8752. [5] C. Picioreanu, I.M. Head, K.P. Katuri, M.C. van Loosdrecht, K. Scott, Water Res. 41 (2007) 2921e2940. [6] S.M. Strycharz-Glaven, R.M. Snider, A. Guiseppi-Elie, L.M. Tender, Energy & Environ. Sci. 4 (2011) 4366. [7] Y. Zeng, Y.F. Choo, B.-H. Kim, P. Wu, J. Power Sources 195 (2010) 79e89. [8] R.A. Rozendal, H.V. Hamelers, R.J. Molenkamp, C.J. Buisman, Water Res. 41 (2007) 1984e1994. [9] C.I. Torres, A. Kato Marcus, B.E. Rittmann, Biotechnol. Bioeng. 100 (2008) 872e881. [10] A.E. Franks, K.P. Nevin, H. Jia, M. Izallalen, T.L. Woodard, D.R. Lovley, Energy & Environ. Sci. 2 (2009) 113e119. [11] J.T. Babauta, H.D. Nguyen, H. Beyenal, Environ. Sci. Technol. 45 (2011) 6654e6660. [12] O. Wanner, W. Gujer, Biotechnol. Bioeng. 28 (1986) 314e328. [13] C. Picioreanu, M. van Loosdrecht, T.P. Curtis, K. Scott, Bioelectrochemistry 78 (2010) 8e24. [14] A. Kato Marcus, C.I. Torres, B.E. Rittmann, Biotechnol. Bioeng. 98 (2007) 1171e1182. [15] S.C. Popat, D. Ki, B.E. Rittmann, C.I. Torres, ChemSusChem 5 (2012) 1071e1079. 169 [16] S.C. Popat, D. Ki, M.N. Young, B.E. Rittmann, C.I. Torres, Buffer pKa and transport govern the concentration overpotential in electrochemical oxygen reduction at neutral pH, ChemElectroChem 1 (2014) 1909e1915, http:// dx.doi.org/10.1002/celc.201402058. [17] Y. Yuan, S. Zhou, J. Tang, Environ. Sci. Technol. 47 (2013) 4911e4917. [18] S. Ou, Y. Zhao, D.S. Aaron, J.M. Regan, M.M. Mench, J. Power Sources 328 (2016) 385e396. [19] S. Ou, H. Kashima, D.S. Aaron, J.M. Regan, M.M. Mench, J. Power Sources 314 (2016) 49e57. [20] A.D. McNaught, A. Wilkinson, Compendium of Chemical Terminology, second ed., Blackwell Science Oxford, 1997. [21] C.I. Torres, A.K. Marcus, P. Parameswaran, B.E. Rittmann, Environ. Sci. Technol. 42 (2008) 6593e6597. [22] K. Broka, P. Ekdunge, J. Appl. Electrochem. 27 (1997) 281e289. [23] M.M. Mench, Fuel Cell Engines, John Wiley & Sons, 2008. [24] Y.A. Gandomi, D. Aaron, T. Zawodzinski, M. Mench, J. Electrochem. Soc. 163 (2016) A5188eA5201. [25] Y.A. Gandomi, D.S. Aaron, M.M. Mench, Electrochimica Acta 218 (2016) 174e190. [26] L. Huang, B.E. Logan, Appl. Microbiol. Biotechnol. 80 (2008) 349e355. € tsch, J. Am. Chem. Soc. 124 (2002) [27] A.E. Kelly, H.D. Ou, R. Withers, V. Do 12013e12019. [28] P.S. Stewart, J. Bacteriol. 185 (2003) 1485e1491. [29] P.G. Falkowski, J.A. Raven, Aquatic Photosynthesis, Princeton University Press, 2013. [30] L. Yuan-Hui, S. Gregory, Geochimica Cosmochimica Acta 38 (1974) 703e714. [31] J. Beun, K. Dircks, M. Van Loosdrecht, J. Heijnen, Water Res. 36 (2002) 1167e1180. [32] R.N. Goldberg, N. Kishore, R.M. Lennen, J. Phys. Chem. Reference Data 31 (2002) 231e370. [33] N.N. Greenwood, A. Earnshaw, Chemistry of the Elements, Elsevier, 1997. € berg, H. Wanner, [34] K.J. Powell, P.L. Brown, R.H. Byrne, T. Gajda, G. Hefter, S. Sjo Pure Appl. Chem. 77 (2005) 739e800.