prediction of concentration profiles of contaminants in groundwater
... The average concentrations of chlorides and heavy metals in leachate from Bhalaswa landfill site and in the groundwater samples at varying radial distances from the landfill are shown in Table 1. The average concentration of chlorides in the leachates from the Bhalaswa landfill site has been found t ...
... The average concentrations of chlorides and heavy metals in leachate from Bhalaswa landfill site and in the groundwater samples at varying radial distances from the landfill are shown in Table 1. The average concentration of chlorides in the leachates from the Bhalaswa landfill site has been found t ...
5548-4.pdf
... orders of magnitude. When the coatings contain predominantly y phase, the error will be low as variation of interdiffusion coefficient with composition is not high. However, when the h phase is formed by heat treatment, the assumption can introduce appreciable error. Hence, Sarkhel and Seigle [4], i ...
... orders of magnitude. When the coatings contain predominantly y phase, the error will be low as variation of interdiffusion coefficient with composition is not high. However, when the h phase is formed by heat treatment, the assumption can introduce appreciable error. Hence, Sarkhel and Seigle [4], i ...
637_diffusion
... • So far only physical processes responsible for the dispersion of a cloud or a plume due to only velocity fluctuations (instantaneous or continuous source in idealized stationary, homogeneous turbulence) • Because of the inherently random character of atmospheric motions, one can never predict with ...
... • So far only physical processes responsible for the dispersion of a cloud or a plume due to only velocity fluctuations (instantaneous or continuous source in idealized stationary, homogeneous turbulence) • Because of the inherently random character of atmospheric motions, one can never predict with ...
Analytical solution for electrolyte concentration distribution in lithium
... the case that the pore wall flux, Ji , is constant across the electrode. If the fluxes of lithium are allowed to change with position only, Wi ðXÞ would have to be modified. Furthermore, if these fluxes are allowed to vary with time, the form of both Vi ðsÞ and Wi ðXÞ would be adjusted. However, if ...
... the case that the pore wall flux, Ji , is constant across the electrode. If the fluxes of lithium are allowed to change with position only, Wi ðXÞ would have to be modified. Furthermore, if these fluxes are allowed to vary with time, the form of both Vi ðsÞ and Wi ðXÞ would be adjusted. However, if ...
Non-equilibrium molecular dynamics algorithm for the calculation of
... 0026-8976/91 $3.00 9 199! Taylor & Francis Ltd ...
... 0026-8976/91 $3.00 9 199! Taylor & Francis Ltd ...
1 3. Molecular mass transport 3.1 Introduction to mass transfer 3.2
... The total mass concentration density ρ is the sum of the total mass of the mixture in unit volume: ...
... The total mass concentration density ρ is the sum of the total mass of the mixture in unit volume: ...
Kinetics and Diffusion Basic concepts in kinetics
... Arrhenius equation can be applied to a wide range of thermally activated processes, including diffusion that we consider next. * Arrhenius equation was first formulated by J. J. Hood on the basis of his studies of the variation of rate constants of some reactions with temperature. Arrhenius demonstr ...
... Arrhenius equation can be applied to a wide range of thermally activated processes, including diffusion that we consider next. * Arrhenius equation was first formulated by J. J. Hood on the basis of his studies of the variation of rate constants of some reactions with temperature. Arrhenius demonstr ...
Particle Size Dependence of the Ionic Diffusivity
... tional theory as implemented in the Vienna Ab Initio Simulation Package (VASP). Elastic band calculations were performed in supercells containing up to eight unit cells and four images in the dilute vacancy limit. Various migration paths conforming to Li+ circumventing FeLi• were considered, and it ...
... tional theory as implemented in the Vienna Ab Initio Simulation Package (VASP). Elastic band calculations were performed in supercells containing up to eight unit cells and four images in the dilute vacancy limit. Various migration paths conforming to Li+ circumventing FeLi• were considered, and it ...
Full Text PDF
... emission at 2.7 eV from pure ZnSe is observed in this case. The edge luminescence is completely thermally quenched at temperatures higher than 200 K. The line of highest photon energy remains unquenched up to room temperature in all Mg-diffused samples. The half-width of this line at T = 10 K is equ ...
... emission at 2.7 eV from pure ZnSe is observed in this case. The edge luminescence is completely thermally quenched at temperatures higher than 200 K. The line of highest photon energy remains unquenched up to room temperature in all Mg-diffused samples. The half-width of this line at T = 10 K is equ ...
The Diffusion Equation A Multi
... particles immersed in a fluctuating (Brownian) environment, the movement of each individual particle is not governed by the diffusion equation. However, many identical particles each obeying the same boundary and initial conditions share statistical properties of their spatial and temporal evolution ...
... particles immersed in a fluctuating (Brownian) environment, the movement of each individual particle is not governed by the diffusion equation. However, many identical particles each obeying the same boundary and initial conditions share statistical properties of their spatial and temporal evolution ...
Power Point Slides P..
... one region to that of the other • slightly disordered • low density in grain boundaries – high mobility – high diffusivity – high chemical reactivity High energy locations where impurities tend to segregate to ...
... one region to that of the other • slightly disordered • low density in grain boundaries – high mobility – high diffusivity – high chemical reactivity High energy locations where impurities tend to segregate to ...
DIFFUSION IN SOLIDS
... conduction in the direction of decreasing temperature, and momentum is transferred in viscous flow in the direction of decreasing velocity. Eq. (12.1) and (12.2) are convenient forms of the rate equation when the density of the total solution is uniform (in solid or liquid solutions, or in ...
... conduction in the direction of decreasing temperature, and momentum is transferred in viscous flow in the direction of decreasing velocity. Eq. (12.1) and (12.2) are convenient forms of the rate equation when the density of the total solution is uniform (in solid or liquid solutions, or in ...
Presentation453.06
... When a solution is at equilibrium the concentration of solute is uniform throughout. If the solute concentration is not uniform, a solute concentration exits that must be reduced to zero if the system is to attain equilibrium Diffusion is the process whereby concentration gradients in a solution are ...
... When a solution is at equilibrium the concentration of solute is uniform throughout. If the solute concentration is not uniform, a solute concentration exits that must be reduced to zero if the system is to attain equilibrium Diffusion is the process whereby concentration gradients in a solution are ...
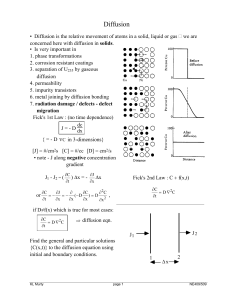
Diffusion
... D ⇒ atomic jump distance & frequency ⇔ random walk theory (text, Eq.7.25) Another way to do this is (see notes) : (no specific micromechanism !) Consider two adjacent crystal lattice planes 1 and 2 [Fig.] separated by α. a {if the planes are {110} type α = 2 since the direction x then is <110> type} ...
... D ⇒ atomic jump distance & frequency ⇔ random walk theory (text, Eq.7.25) Another way to do this is (see notes) : (no specific micromechanism !) Consider two adjacent crystal lattice planes 1 and 2 [Fig.] separated by α. a {if the planes are {110} type α = 2 since the direction x then is <110> type} ...
CHAPTER 5 Carrier Transport Phenomena
... • Describe the mechanism of carrier drift and induced drift current due to an applied electric field. • Define and describe the characteristics of carrier mobility. • Describe the mechanism of carrier diffusion and induced diffusion current due to a gradient in the carrier concentration. • Define th ...
... • Describe the mechanism of carrier drift and induced drift current due to an applied electric field. • Define and describe the characteristics of carrier mobility. • Describe the mechanism of carrier diffusion and induced diffusion current due to a gradient in the carrier concentration. • Define th ...
biol 1406 chapter 3: water
... a. potential energy. b. heat energy. c. kinetic energy. d. random energy. 7. The first scientist to observe evidence of the random motion of molecules was a. Brown. b. Darwin. c. Mendel. d. Hooke. 8. The net movement of particles from an area of higher concentration to an area of lower concentration ...
... a. potential energy. b. heat energy. c. kinetic energy. d. random energy. 7. The first scientist to observe evidence of the random motion of molecules was a. Brown. b. Darwin. c. Mendel. d. Hooke. 8. The net movement of particles from an area of higher concentration to an area of lower concentration ...
Lecture 18 More on Diffusion and Kinetic Energy
... This is a particularly simple case! • Single concentration gradient along the x-axis. • Initial state is defined by a simple concentration difference. • Volume has a simple shape. ...
... This is a particularly simple case! • Single concentration gradient along the x-axis. • Initial state is defined by a simple concentration difference. • Volume has a simple shape. ...
Diffusion
Diffusion is the net movement of molecules or atoms from a region of high concentration to a region of low concentration. This is also referred to as the movement of a substance down a concentration gradient. A gradient is the change in the value of a quantity (e.g., concentration, pressure, temperature) with the change in another variable (usually distance). For example, a change in concentration over a distance is called a concentration gradient, a change in pressure over a distance is called a pressure gradient, and a change in temperature over a distance is a called a temperature gradient.The word diffusion is derived from the Latin word, ""diffundere"", which means ""to spread out"" (if a substance is “spreading out”, it is moving from an area of high concentration to an area of low concentration). A distinguishing feature of diffusion is that it results in mixing or mass transport, without requiring bulk motion (bulk flow). Thus, diffusion should not be confused with convection, or advection, which are other transport phenomena that utilize bulk motion to move particles from one place to another.