Making and using alcohol
... into a gas. Volatility increases as boiling point decreases. If you were asked to compare and explain the differences ...
... into a gas. Volatility increases as boiling point decreases. If you were asked to compare and explain the differences ...
Chap Thirteen: Alcohols
... Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target alcohol in high yield. Predict the relative acidity of alcohols within a functional group class and compared to oth ...
... Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target alcohol in high yield. Predict the relative acidity of alcohols within a functional group class and compared to oth ...
Alcohols
... Where “R” represents any chain of carbon and hydrogen atoms. • If there is more than one hydroxyl group, it is called a polyalcohol. They are named almost the same as regular alcohols except you add a “di”, “tri”, etc. before the “ol” ending. For example ...
... Where “R” represents any chain of carbon and hydrogen atoms. • If there is more than one hydroxyl group, it is called a polyalcohol. They are named almost the same as regular alcohols except you add a “di”, “tri”, etc. before the “ol” ending. For example ...
Document
... At room temperature ethanol is a liquid whilst ethane is a gas. Can you explain why? ...
... At room temperature ethanol is a liquid whilst ethane is a gas. Can you explain why? ...
Depending on C, where the
... •e. g. ethanol can be very ecological fuel to engines in the future (but it bond with water and it cause corrosion, however we can dispatch this) •they are very extended in nature in ester forms (e. g. fat, wax, ...
... •e. g. ethanol can be very ecological fuel to engines in the future (but it bond with water and it cause corrosion, however we can dispatch this) •they are very extended in nature in ester forms (e. g. fat, wax, ...
CHAPTER 10 Properties and Preparation of Alcohols
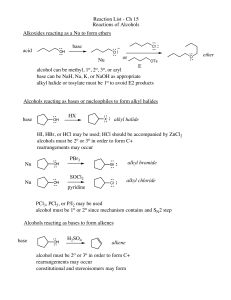
... Synthesis of Alcohols (Review) • Nucleophilic substitution (usually SN2) of alkyl halide. • Alkene Addition: – Water in acid solution (suffers from rearrangements). – Oxymercuration–demercuration. – Hydroboration–oxidation. ...
... Synthesis of Alcohols (Review) • Nucleophilic substitution (usually SN2) of alkyl halide. • Alkene Addition: – Water in acid solution (suffers from rearrangements). – Oxymercuration–demercuration. – Hydroboration–oxidation. ...
Functional Groups
... A specific arrangement of atoms in an organic compound that is capable of characteristic chemical reactions. In other words, a substituent group other than an alkyl group. Most organic chemistry is functionalgroup chemistry. We will do one functional group. ...
... A specific arrangement of atoms in an organic compound that is capable of characteristic chemical reactions. In other words, a substituent group other than an alkyl group. Most organic chemistry is functionalgroup chemistry. We will do one functional group. ...
Carboxylic Acid
... You know Alkanes and Benzenes, and Alkynes and Alkenes, Amines and Alcohols, Aldehydes and Ketones...... But do you recall, the most famous functional group of all Carboxylic Acid, Has a carbonyl and a hydroxyl It loves to donate protons Then an anion is formed Of all the other acids It’s the most c ...
... You know Alkanes and Benzenes, and Alkynes and Alkenes, Amines and Alcohols, Aldehydes and Ketones...... But do you recall, the most famous functional group of all Carboxylic Acid, Has a carbonyl and a hydroxyl It loves to donate protons Then an anion is formed Of all the other acids It’s the most c ...
Oxidation of Alcohols
... • Primary and secondary alcohols can be oxidised using an oxidising agent, notated by [o]. • A suitable oxidising agent is a solution containing acidified dichromate ions (H+ and Cr O 2-). • These ions come from a mixture of K Cr O and sulphuric acid. • During the reaction there will be a colour cha ...
... • Primary and secondary alcohols can be oxidised using an oxidising agent, notated by [o]. • A suitable oxidising agent is a solution containing acidified dichromate ions (H+ and Cr O 2-). • These ions come from a mixture of K Cr O and sulphuric acid. • During the reaction there will be a colour cha ...
Solubility of Alcohols
... By definition, an Alcohol is an organic compound containing a –OH grouping. Each of the above are referred to as n-Alcohols, or Normal Alcohols, as the –OH group occurs at the end of the carbon chain. ...
... By definition, an Alcohol is an organic compound containing a –OH grouping. Each of the above are referred to as n-Alcohols, or Normal Alcohols, as the –OH group occurs at the end of the carbon chain. ...
$doc.title
... Prepared by José Laboy, MS http: www.chem.wisc.edu/areas /clc (Resource page) Reactions of Alcohols #5: Oxidation of Primary Alcohols to Aldehydes ...
... Prepared by José Laboy, MS http: www.chem.wisc.edu/areas /clc (Resource page) Reactions of Alcohols #5: Oxidation of Primary Alcohols to Aldehydes ...
07.Chapter7.Alcohols and Related
... Nucleophilicity of halide ion is not strong enough for SN2 Please refer to page 219 ...
... Nucleophilicity of halide ion is not strong enough for SN2 Please refer to page 219 ...
Group Activity 3 [10 PTS]
... 1. Write the condensed structural formula of each of the following alcohols a. 1-propanol ...
... 1. Write the condensed structural formula of each of the following alcohols a. 1-propanol ...
Alcohol, Aldehydes and Acids
... made from ethene by the addition of water for nonbeverage use, like an additive to gassoline to make "gasahol." 2-Propanol (better known as isopropyl alcohol) is in (with some water) rubbing alcohol. It is also used in gasoline to prevent freezing of the gas line in automobiles by keeping excess moi ...
... made from ethene by the addition of water for nonbeverage use, like an additive to gassoline to make "gasahol." 2-Propanol (better known as isopropyl alcohol) is in (with some water) rubbing alcohol. It is also used in gasoline to prevent freezing of the gas line in automobiles by keeping excess moi ...
Slide 1 - MrFisherChemistry
... The hydration of ethene is used to make alcohols by using fractions of distilled crude oil from fractioning columns. It is made in the presence of an acid catalyst, normally phosphoric acid, and it needs high temperature and pressure. ...
... The hydration of ethene is used to make alcohols by using fractions of distilled crude oil from fractioning columns. It is made in the presence of an acid catalyst, normally phosphoric acid, and it needs high temperature and pressure. ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.



















![Group Activity 3 [10 PTS]](http://s1.studyres.com/store/data/010780770_1-3445600a9b56e890a0f283c789afe8fb-300x300.png)



