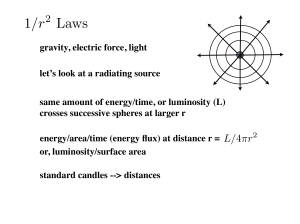
Modern Model of the Atom
... CONFIGURATIONS. The rules that govern the way the electrons fill the atomic orbitals are: 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic orbital may hold at most two electrons. Each must have an opposite spin. 3. HUND’S RULE ...
... CONFIGURATIONS. The rules that govern the way the electrons fill the atomic orbitals are: 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic orbital may hold at most two electrons. Each must have an opposite spin. 3. HUND’S RULE ...
form revision a
... temperature. Only ionic compounds can conduct electricity, they can only do this when molten or in solution. ...
... temperature. Only ionic compounds can conduct electricity, they can only do this when molten or in solution. ...
The Quantum Mechanical Behavior of Light and Matter
... shorter wavelength photons have more energy, momentum --> generate faster moving electrons higher intensity light generates more electrons, not higher velocity electrons ...
... shorter wavelength photons have more energy, momentum --> generate faster moving electrons higher intensity light generates more electrons, not higher velocity electrons ...
Nuclear and Particle Physics
... Clear experimental evidence that atoms contain electrons – where are they? Heisenberg ⇒ simplest atom = H ...
... Clear experimental evidence that atoms contain electrons – where are they? Heisenberg ⇒ simplest atom = H ...
Atomic Structure Origin of the elements Structure of atoms Periodic Trends
... ❧ rapidly decay to form stable neutron rich nuclei • P process ❧ Proton capture, not as common ...
... ❧ rapidly decay to form stable neutron rich nuclei • P process ❧ Proton capture, not as common ...
Bohr Model and Quantum Model
... may know the location of an electron or the velocity of electron but you may not know both simultaneously ...
... may know the location of an electron or the velocity of electron but you may not know both simultaneously ...
Chapter 4 Arrangement of Electrons in Atoms
... • Explain how the Heisenberg uncertainty principle and the Schrödinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers and describe their significance. • Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per ...
... • Explain how the Heisenberg uncertainty principle and the Schrödinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers and describe their significance. • Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per ...
Zumdahl`s Chapter 7
... – Cleave space with an x=0 plane – But y=0 or z=0 work as well, so there are three or 2l+1 suborbitals. – The ml sequence always gives 2l+1 – ml differentiates directions in space for chemical bonding! ...
... – Cleave space with an x=0 plane – But y=0 or z=0 work as well, so there are three or 2l+1 suborbitals. – The ml sequence always gives 2l+1 – ml differentiates directions in space for chemical bonding! ...
Chemistry Name______________________________________
... 2. What is an atom's ground state? 3. What are two reasons given for why Bohr's model was not correct? ...
... 2. What is an atom's ground state? 3. What are two reasons given for why Bohr's model was not correct? ...
Final Exam Review
... 111. classify each of the following as a physical change or a chemical change: A. an aspirin tablet is crushed to a powder B. a red rose turns brown C. grape juice turns to wine D. fingernail polish remover evaporates E. a bean seed sprouts F. a piece of copper is beaten into a thin sheet 116. how m ...
... 111. classify each of the following as a physical change or a chemical change: A. an aspirin tablet is crushed to a powder B. a red rose turns brown C. grape juice turns to wine D. fingernail polish remover evaporates E. a bean seed sprouts F. a piece of copper is beaten into a thin sheet 116. how m ...
Atomic Spectra Bohr Model Notes
... Helped lay the foundation for modern quantum theory (atomic model). ...
... Helped lay the foundation for modern quantum theory (atomic model). ...
AS Physics
... “Number of protons in the nucleus (also equal to number of electrons)” Nucleon number or Mass number “Number of protons and neutrons in an atom’s nucleus” Isotope “A form of an element with the same proton number but different neutron number” ...
... “Number of protons in the nucleus (also equal to number of electrons)” Nucleon number or Mass number “Number of protons and neutrons in an atom’s nucleus” Isotope “A form of an element with the same proton number but different neutron number” ...
Physical Chemistry
... electrochemistry that amounts of electric current proportional to amounts of some substances could be liberated in an electrolytic cell. The term “electron” was suggested as a natural “unit” of electricity. » But Thomson experimentally observes electrons as particles with charge & mass. ...
... electrochemistry that amounts of electric current proportional to amounts of some substances could be liberated in an electrolytic cell. The term “electron” was suggested as a natural “unit” of electricity. » But Thomson experimentally observes electrons as particles with charge & mass. ...