Modern Physics
... from the nucleus for the hydrogen atom is 2 ao. Find the probability of finding the 1-s electron at a distance greater than 2 ao according to quantum mechanics. ...
... from the nucleus for the hydrogen atom is 2 ao. Find the probability of finding the 1-s electron at a distance greater than 2 ao according to quantum mechanics. ...
107 chem Assement Q
... 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 state to the n = 2 state. 6. “It is impos ...
... 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 state to the n = 2 state. 6. “It is impos ...
Chemistry Unit Study Guide Key
... 2) examples of compounds – CO2, C6H12O6, NaCl, N2, O2, Fe2O3, H2O 3) where metals and nonmetals are found on the periodic table – Metals are to the left of the zig-zag line; Non-metals are to the right. 4) examples of physical and chemical properties – Physical Properties: Luster; Hardness; Color; C ...
... 2) examples of compounds – CO2, C6H12O6, NaCl, N2, O2, Fe2O3, H2O 3) where metals and nonmetals are found on the periodic table – Metals are to the left of the zig-zag line; Non-metals are to the right. 4) examples of physical and chemical properties – Physical Properties: Luster; Hardness; Color; C ...
The Quantum Atom (section 18)
... Allday, p784 Muncaster). Particles given off from cathode always have the same charge to mass ratio, no matter what element the cathode is made of. Conclusion – they are subatomic particles. Thomson proposes plum pudding model. Diffuse positive charge with embedded electrons. Rutherford, Geiger and ...
... Allday, p784 Muncaster). Particles given off from cathode always have the same charge to mass ratio, no matter what element the cathode is made of. Conclusion – they are subatomic particles. Thomson proposes plum pudding model. Diffuse positive charge with embedded electrons. Rutherford, Geiger and ...
Matter and Energy Identify a chemical physical change Identify a
... Part 2 – answer the following 1. Describe Rutherford’s gold foil experiment, his conclusions, and his model of the atom. 2. Draw Thomson’s model of the atom. 3. How did the Dalton’s model of the atom look? Why did it look like that? Part 3 – multiple choice 1. Today’s model of the atom is due to a. ...
... Part 2 – answer the following 1. Describe Rutherford’s gold foil experiment, his conclusions, and his model of the atom. 2. Draw Thomson’s model of the atom. 3. How did the Dalton’s model of the atom look? Why did it look like that? Part 3 – multiple choice 1. Today’s model of the atom is due to a. ...
Quantum Mechanics
... Discharge tubes are used that contains very little gas at low pressure A high voltage is applied across the atoms, which cause them to interact to create light (one of the four interactions of photons) For hydrogen, there is an equation for wavelength of light emitted ...
... Discharge tubes are used that contains very little gas at low pressure A high voltage is applied across the atoms, which cause them to interact to create light (one of the four interactions of photons) For hydrogen, there is an equation for wavelength of light emitted ...
LT1: Electron.NOTES - Simpson County Schools
... What is the quantum mechanical model of the atom and how is it different from the Bohr model? _________________________________________________________________________________________________________ ____________________________________________________________________________________________________ ...
... What is the quantum mechanical model of the atom and how is it different from the Bohr model? _________________________________________________________________________________________________________ ____________________________________________________________________________________________________ ...
Introduction to Chemistry
... Learning Objectives (from Zumdahl Resource Guide): (3-4 days lecture/discussion) To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffract ...
... Learning Objectives (from Zumdahl Resource Guide): (3-4 days lecture/discussion) To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffract ...
Dalton`s Atomic Theory
... John Dalton (in 1805) proposes his Atomic Theory to explain the results of the quantitative studies of several scientists (including Lavoisier, Proust, and himself, among many others). Dalton’s Atomic Theory a. Elements consist of tiny, indivisible particles called atoms. b. All the atoms of a given ...
... John Dalton (in 1805) proposes his Atomic Theory to explain the results of the quantitative studies of several scientists (including Lavoisier, Proust, and himself, among many others). Dalton’s Atomic Theory a. Elements consist of tiny, indivisible particles called atoms. b. All the atoms of a given ...
Prentice Hall Chemistry Worksheets
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
5 ELECTRONS IN ATOMS Vocabulary Review Name ___________________________
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
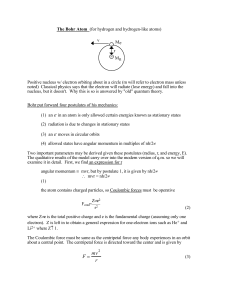
lect10
... which the electrons orbit the nucleus in accordance with Newton’s Laws but do not radiate energy an atom emits or absorbs energy only when an electron moves from one stable state to another ...
... which the electrons orbit the nucleus in accordance with Newton’s Laws but do not radiate energy an atom emits or absorbs energy only when an electron moves from one stable state to another ...
Ch.5 VocabReview
... Choose the term from the following list that best matches each description. quantum photons hertz Pauli exclusion principle wavelength ...
... Choose the term from the following list that best matches each description. quantum photons hertz Pauli exclusion principle wavelength ...
IB HL Physics More Problems on Quantum and Nuclear Physics_
... 4. A proton and an alpha particle have the same de Broglie wavelength. Which of the following is approximately the ratio ...
... 4. A proton and an alpha particle have the same de Broglie wavelength. Which of the following is approximately the ratio ...
Modern Physics Important Concepts for AP Test
... o E = hf = (hc)/λ o p = E/c = h/ λ Matter equations (Matter does not move at c, do not use c = λּ f) o deBroglie Wavelength λ = h/p = h/(mv) (Common problem on exam) o f = E/h frequency of matter waves Davisson Germer Experiment measured wavelength of electrons. (wave properties of matter) o Fir ...
... o E = hf = (hc)/λ o p = E/c = h/ λ Matter equations (Matter does not move at c, do not use c = λּ f) o deBroglie Wavelength λ = h/p = h/(mv) (Common problem on exam) o f = E/h frequency of matter waves Davisson Germer Experiment measured wavelength of electrons. (wave properties of matter) o Fir ...