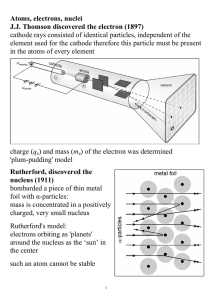
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... Davisson and Germer (1927) used electron beams to induce diffraction through a thin metal foil: interference interference phenomena have been shown with various other particles: duality is a general characteristic of matter Propagation law of free electrons state function ψ(x,t); we can ‘find’ the e ...
... Davisson and Germer (1927) used electron beams to induce diffraction through a thin metal foil: interference interference phenomena have been shown with various other particles: duality is a general characteristic of matter Propagation law of free electrons state function ψ(x,t); we can ‘find’ the e ...
Steve Hansen`s second test - Kwantlen Polytechnic University
... Instructions: There are 29 questions on this exam. Part A consists of multiple choice questions and Part B is a problem solving section. ALL WORK MUST BE SHOWN IN PART B TO RECEIVE ANY CREDIT. A periodic chart is included with this exam. Rough Calculations may be done on the back side of a page. Max ...
... Instructions: There are 29 questions on this exam. Part A consists of multiple choice questions and Part B is a problem solving section. ALL WORK MUST BE SHOWN IN PART B TO RECEIVE ANY CREDIT. A periodic chart is included with this exam. Rough Calculations may be done on the back side of a page. Max ...
Radioisotopes
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
... • Isotopes are any of the different types of atoms (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have di ...
Development of the Model of the Atom
... around an atomic nucleus. Experiments demonstrated that electrons, like light waves, can be bent or diffracted (bending as it passes through a small opening). Also, some experiments showed that electron beams interfere with each other. ...
... around an atomic nucleus. Experiments demonstrated that electrons, like light waves, can be bent or diffracted (bending as it passes through a small opening). Also, some experiments showed that electron beams interfere with each other. ...
TERM 2 Unit 3 YR 9 SCI It is elementary
... 9 SCIENCE – Term 2 Unit 3: IT’S ELEMENTARY (5 WEEKS) ...
... 9 SCIENCE – Term 2 Unit 3: IT’S ELEMENTARY (5 WEEKS) ...
7.4 The Wave Nature of Matter * 7.5 Quantum Mechanics and the Atom
... The Shapes of Atomic Orbitals • The shapes of atomic orbitals are important because covalent chemical bonds depend on sharing the electrons that occupy these orbitals. • A bond consists of the overlap of atomic orbitals on adjacent atoms. • The shape of overlapping orbitals determines the shape of ...
... The Shapes of Atomic Orbitals • The shapes of atomic orbitals are important because covalent chemical bonds depend on sharing the electrons that occupy these orbitals. • A bond consists of the overlap of atomic orbitals on adjacent atoms. • The shape of overlapping orbitals determines the shape of ...
DEVELOPMENT OF THE ATOMIC THEORY PROJECT due Friday
... In succeeding layers, you will add detail to your atom and name the scientist who developed this part of the atomic theory. You will also include a picture or a brief description of the method the scientist used. You must include development of the quantum model in your depiction of the electron clo ...
... In succeeding layers, you will add detail to your atom and name the scientist who developed this part of the atomic theory. You will also include a picture or a brief description of the method the scientist used. You must include development of the quantum model in your depiction of the electron clo ...
2·QUIZLET VOCABULARY: Quantum Numbers Study online at
... electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) quantum Number: ml Indicates orientation of orbital in space S- 1 orbital P- 3 orbitals D- 5 orbitals F- 7 orbitals 6. orbital: A 3-D space around the nucleus where an electron is likely (high proba ...
... electron, and all electrons in singly occupied orbitals must have the same spin 5. Magnetic (orbital) quantum Number: ml Indicates orientation of orbital in space S- 1 orbital P- 3 orbitals D- 5 orbitals F- 7 orbitals 6. orbital: A 3-D space around the nucleus where an electron is likely (high proba ...
Chemistry Science Notebook
... The quantum concept concludes that matter can gain or lose only in small, specific amounts called ...
... The quantum concept concludes that matter can gain or lose only in small, specific amounts called ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... Why do atoms exhibit discontinuous (line) spectra when they emit light? Why can’t an atom emit any wavelength of light? Energy is quantized. Emission is due to specific transitions between ground and excited states. 18. Refer to the activity series in chapter 10. For the single replacement reactions ...
... Why do atoms exhibit discontinuous (line) spectra when they emit light? Why can’t an atom emit any wavelength of light? Energy is quantized. Emission is due to specific transitions between ground and excited states. 18. Refer to the activity series in chapter 10. For the single replacement reactions ...
Chapter 5 Electrons In Atoms 5.1 Models of the Atom The
... Each energy sublevel corresponds to an orbital of a different shape, which describes where the _____________________ is likely to be found. Four of the five kinds of d orbitals have clover leaf shapes. The lowest principal energy level (n=1) has only one sublevel, called___________ The number of el ...
... Each energy sublevel corresponds to an orbital of a different shape, which describes where the _____________________ is likely to be found. Four of the five kinds of d orbitals have clover leaf shapes. The lowest principal energy level (n=1) has only one sublevel, called___________ The number of el ...