Section 12.2 - CPO Science
... you come from this property of elements to emit or absorb light of only certain colors. ...
... you come from this property of elements to emit or absorb light of only certain colors. ...
Day 19: Electrostatic Potential Energy & CRT Applications
... electrons emitted by the cathode are accelerated by a high voltage anode, through a small hole in the anode – thus coins the term “electron gun” ...
... electrons emitted by the cathode are accelerated by a high voltage anode, through a small hole in the anode – thus coins the term “electron gun” ...
SCH3U Course Review
... Ionization energies tend to increase with increasing atomic radii decrease with increasing nuclear charge decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
... Ionization energies tend to increase with increasing atomic radii decrease with increasing nuclear charge decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
Midterm Review File
... 25. If an atom has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2, how many valence electrons does it have? ...
... 25. If an atom has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2, how many valence electrons does it have? ...
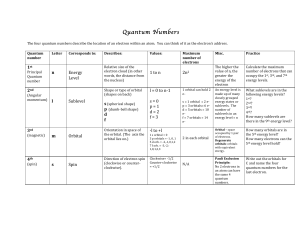
CHAPTER 5 NOTES – ELECTRONS IN ATOMS
... • Energy Levels – the fixed energies an electron can have – like rungs of a ladder • Quantum – the amount of energy required to move an electron from one energy level to another energy level • Quantum Mechanical Model – the modern description of the electron in atoms – from the mathematical solutio ...
... • Energy Levels – the fixed energies an electron can have – like rungs of a ladder • Quantum – the amount of energy required to move an electron from one energy level to another energy level • Quantum Mechanical Model – the modern description of the electron in atoms – from the mathematical solutio ...
105 photoelectric_calc
... • Photon Q has just enough energy to cause photoemission. There is no ‘spare energy’ left over, so the photoelectrons have no KE – all the energy went into leaving the surface. This is of course the work function, so the work function of this particular metal is 4 eV. • Photon R had 6 eV. 4 eV of th ...
... • Photon Q has just enough energy to cause photoemission. There is no ‘spare energy’ left over, so the photoelectrons have no KE – all the energy went into leaving the surface. This is of course the work function, so the work function of this particular metal is 4 eV. • Photon R had 6 eV. 4 eV of th ...
Notes-15 - KSU Physics
... electron falling down to fill the hole, and another electron gets emitted, in order to conserve the total energy. The emitted electron is called an Auger electron. The stabilization can also be achieved by emitting a radiation, or photon. This is the source of characteristic X-rays. Based on the she ...
... electron falling down to fill the hole, and another electron gets emitted, in order to conserve the total energy. The emitted electron is called an Auger electron. The stabilization can also be achieved by emitting a radiation, or photon. This is the source of characteristic X-rays. Based on the she ...
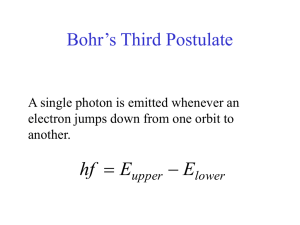
Atomic Spectroscopy and the Bohr Model
... c 2.998X108 m/s ν = --- = --------------------- = 5.82X1014 Hz λ 5.15X10-7 m ...
... c 2.998X108 m/s ν = --- = --------------------- = 5.82X1014 Hz λ 5.15X10-7 m ...
Quantum Physics Cumulative Review
... 1. How was Einstein able to apply Planck’s idea that light waves had quantized energy to explain why some wavelengths of light could knock electrons off a block of a particular metal and create a photocurrent and others couldn’t? 2. How does the law of Conservation of Energy apply to a light beam hi ...
... 1. How was Einstein able to apply Planck’s idea that light waves had quantized energy to explain why some wavelengths of light could knock electrons off a block of a particular metal and create a photocurrent and others couldn’t? 2. How does the law of Conservation of Energy apply to a light beam hi ...
Chapter 30: Quantum Physics Chapter 31: Atomic Physics Chapter
... Planck’s theory of blackbody radiation implies a one-to-one relationship between the absolute temperature of a blackbody and the frequency of light at the peak of its radiated energy spectrum. This relationship is given by Wien’s displacement law (Equation 30-1). Therefore, by measuring the peak in ...
... Planck’s theory of blackbody radiation implies a one-to-one relationship between the absolute temperature of a blackbody and the frequency of light at the peak of its radiated energy spectrum. This relationship is given by Wien’s displacement law (Equation 30-1). Therefore, by measuring the peak in ...
FIZICA
... S10. The Pauli principle states that: Into an atom (molecule) there are no two electrons (in general fermions) characterized by identical quantum numbers. Name these four quantum numbers and calculate the maximum number of orbitals for a hidrogenoid atom with two electrons characterized by i) n1 = 2 ...
... S10. The Pauli principle states that: Into an atom (molecule) there are no two electrons (in general fermions) characterized by identical quantum numbers. Name these four quantum numbers and calculate the maximum number of orbitals for a hidrogenoid atom with two electrons characterized by i) n1 = 2 ...
Unit 2
... D. covalent bond. 61. The electrons available to be lost, gained, or shared in the formation of chemical compounds are referred to as _ A. ions. B. electron clouds. C. d electrons. D. valence electrons. 62. In many compounds, atoms of main-group elements form bonds so that the number of electrons in ...
... D. covalent bond. 61. The electrons available to be lost, gained, or shared in the formation of chemical compounds are referred to as _ A. ions. B. electron clouds. C. d electrons. D. valence electrons. 62. In many compounds, atoms of main-group elements form bonds so that the number of electrons in ...
Lecture 3 Teaching notes
... wavefunctions for one particle, and the total wavefunction for all the electrons of a many-electron system. I will try to distinguish these two senses by speaking of a “configuration” of a many-particle system when there is a chance of confusion. The lowest-energy configuration state of the system i ...
... wavefunctions for one particle, and the total wavefunction for all the electrons of a many-electron system. I will try to distinguish these two senses by speaking of a “configuration” of a many-particle system when there is a chance of confusion. The lowest-energy configuration state of the system i ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.