Simple Harmonic Oscillator
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
Introduction to Spectroscopy
... • Only in-phase motion (purely elastic) and can have resonance when ω→ωo so that amplitude grows • Since x2 goes to 0, we can connect it with damping or energy loss ...
... • Only in-phase motion (purely elastic) and can have resonance when ω→ωo so that amplitude grows • Since x2 goes to 0, we can connect it with damping or energy loss ...
Atomic Spectra - Northeast High School
... An atom’s only electron is in the fourth energy level (-2.35 eV). How many different photon energies can be emitted as this photon returns to the ground state (-15.8 eV)? What is the frequency of the photon that would be emitted if the electron returned to the ground state in a single transition? ...
... An atom’s only electron is in the fourth energy level (-2.35 eV). How many different photon energies can be emitted as this photon returns to the ground state (-15.8 eV)? What is the frequency of the photon that would be emitted if the electron returned to the ground state in a single transition? ...
PS7aChemistryReviewRevised
... Alcohol boils. Paint dries. A photosynthesizing plant produces sugar. ...
... Alcohol boils. Paint dries. A photosynthesizing plant produces sugar. ...
lecture 5 radiation and matter
... transition. There is no ``in-between'‘ in quantum descriptions of matter energy interactions. ...
... transition. There is no ``in-between'‘ in quantum descriptions of matter energy interactions. ...
Solution - UMD Physics
... 2000 times larger. Decide whether their associated magnetic moment is (greater than/less than/ equal to) the electron magnetic moment µB, and explain why. (3) ...
... 2000 times larger. Decide whether their associated magnetic moment is (greater than/less than/ equal to) the electron magnetic moment µB, and explain why. (3) ...
Chem 400 Chem 150 REVIEW SHEET Amanda R
... o Atomic radii increases to the left and down o Electron Affinity/Ionization Energy and electronegativity increases going up and to the right Types of Bonds – must know which bond types can form and how o Covalent o Ionic o Molecular o Bond order # of bonding e- - # of antibonding e-/2 Stoichiometry ...
... o Atomic radii increases to the left and down o Electron Affinity/Ionization Energy and electronegativity increases going up and to the right Types of Bonds – must know which bond types can form and how o Covalent o Ionic o Molecular o Bond order # of bonding e- - # of antibonding e-/2 Stoichiometry ...
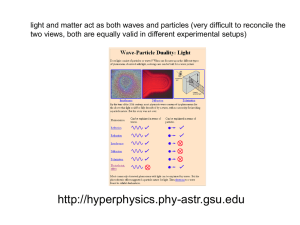
L 35 Modern Physics [1]
... Einstein explains the PE effect, receives Nobel Prize in 1921 • Light is an electromagnetic wave, but when it interacts the metal it behaves like a particle, a light particle called a photon. • A beam of light is a beam of photons. ...
... Einstein explains the PE effect, receives Nobel Prize in 1921 • Light is an electromagnetic wave, but when it interacts the metal it behaves like a particle, a light particle called a photon. • A beam of light is a beam of photons. ...
chemistry - cloudfront.net
... Then I would look at the topic statement below (the capital letter phrases) for the first topic. Find this topic and all related information or materials in your possession and study them thoroughly! I would suggest you re-read your textbook/notes and re-work old problems. When you feel you have the ...
... Then I would look at the topic statement below (the capital letter phrases) for the first topic. Find this topic and all related information or materials in your possession and study them thoroughly! I would suggest you re-read your textbook/notes and re-work old problems. When you feel you have the ...
IB HL Physics More Problems on Quantum and Nuclear Physics_
... 4. A proton and an alpha particle have the same de Broglie wavelength. Which of the following is approximately the ratio ...
... 4. A proton and an alpha particle have the same de Broglie wavelength. Which of the following is approximately the ratio ...
PHY4605–Introduction to Quantum Mechanics II Spring 1997 Problem Set 4 Jan. 31, 2005
... classical physics (Gauss’s law) to find the classical potential V1 (r) associated with the charge density above, and define the perturbation to the point-proton model to be δV (r) = V1 (r) − V0 (r). Use 1st-order perturbation theory to find the correction δE0 to the ground state energy due to the fi ...
... classical physics (Gauss’s law) to find the classical potential V1 (r) associated with the charge density above, and define the perturbation to the point-proton model to be δV (r) = V1 (r) − V0 (r). Use 1st-order perturbation theory to find the correction δE0 to the ground state energy due to the fi ...
3.4oquantum.4u
... we cannot specify exact orbits. Another problem is when an electron changes energy levels during the emission of atomic spectra. ...
... we cannot specify exact orbits. Another problem is when an electron changes energy levels during the emission of atomic spectra. ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.










![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/008517000_1-9aef89c0ca089782f518550164188024-300x300.png)