Chemical Bonds
... An ion is an atom or molecule with a net electric charge (either positive or negative) due to the loss or gain of one or more electrons. ...
... An ion is an atom or molecule with a net electric charge (either positive or negative) due to the loss or gain of one or more electrons. ...
BAND THEORY OF SOLIDS
... A solid contains an enormous number of atoms packed closely together. Each atom, when isolated, has a discrete set of electron energy levels 1s,2s,2p,....... If we imagine all the N atoms of the solid to be isolated from one another, they would have completely coinciding schemes of their energy leve ...
... A solid contains an enormous number of atoms packed closely together. Each atom, when isolated, has a discrete set of electron energy levels 1s,2s,2p,....... If we imagine all the N atoms of the solid to be isolated from one another, they would have completely coinciding schemes of their energy leve ...
Scale, structure and behaviour
... Sources: http://www.apt owders.com/images/zno/im_zinc_oxide_particles.jpg http://www.abc.net.au/science/news/stories/s1165709.htm ; http://www.4girls.gov/body/sunscreen.jpg ...
... Sources: http://www.apt owders.com/images/zno/im_zinc_oxide_particles.jpg http://www.abc.net.au/science/news/stories/s1165709.htm ; http://www.4girls.gov/body/sunscreen.jpg ...
Heisenberg uncertainty principle
... He proposed that a cat be placed in a sealed box. The release of a poison is then subject to the probabilistic decay of a radioactive isotope. If the isotope decays, the poison is released. If no decay occurs, the poison is not released. The result is that the cat is in a superposition of states ...
... He proposed that a cat be placed in a sealed box. The release of a poison is then subject to the probabilistic decay of a radioactive isotope. If the isotope decays, the poison is released. If no decay occurs, the poison is not released. The result is that the cat is in a superposition of states ...
Chapter 2 - Las Positas College
... intermediate energy state then the energy of the emitted photon is less than the energy of the colliding electron that boosted up the orbital electron. In this case ∆Eelec > Ephoton. Both cases are possible, so the correct choice is B. ...
... intermediate energy state then the energy of the emitted photon is less than the energy of the colliding electron that boosted up the orbital electron. In this case ∆Eelec > Ephoton. Both cases are possible, so the correct choice is B. ...
The Egyptian American International School
... 9.2 Using Chemical Equations to Calculate Mass To calculate masses from the moles of reactants needed or products formed, we can use the molar masses of substances for finding the masses (g) needed or formed. 9.3 Limiting Reactants and Percent Yield Often, reactants in a chemical reaction are n ...
... 9.2 Using Chemical Equations to Calculate Mass To calculate masses from the moles of reactants needed or products formed, we can use the molar masses of substances for finding the masses (g) needed or formed. 9.3 Limiting Reactants and Percent Yield Often, reactants in a chemical reaction are n ...
Chapter 5 Electrons in Atoms
... The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. ...
... The propeller blade has the same probability of being anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. ...
PHY 410 Final Examination, Spring 2008 April 30, 2008 (5:45-7:45 p.m.)
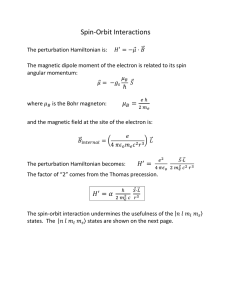
... external magnetic field B, the energy levels are given by E ( m) = ∆ m 2 − µmB , where ∆ is a constant energy depending on the crystal, and µ m is the magnetic moment associated with the state m . (15 points) a) Write down the partition function. b) What is the average magnetic moment M of the atom? ...
... external magnetic field B, the energy levels are given by E ( m) = ∆ m 2 − µmB , where ∆ is a constant energy depending on the crystal, and µ m is the magnetic moment associated with the state m . (15 points) a) Write down the partition function. b) What is the average magnetic moment M of the atom? ...
IB Phys..
... – 1. Low pressure gas is energized by applying a potential difference across it causing it to heat up. – 2. The hot gas emits light energy only at certain well-defined frequencies, as seen through a diffraction grating (spectroscope) or prism. ...
... – 1. Low pressure gas is energized by applying a potential difference across it causing it to heat up. – 2. The hot gas emits light energy only at certain well-defined frequencies, as seen through a diffraction grating (spectroscope) or prism. ...
Notes - Photons, the Photoelectric Effect and the Compton Effect (ppt)
... • Classical physics predicts that any frequency of light can eject electrons as long as the intensity is high enough. • Experimental data shows there is a minimum (cutoff frequency) that the light must have. • Classical physics predicts that the kinetic energy of the ejected electrons should increas ...
... • Classical physics predicts that any frequency of light can eject electrons as long as the intensity is high enough. • Experimental data shows there is a minimum (cutoff frequency) that the light must have. • Classical physics predicts that the kinetic energy of the ejected electrons should increas ...
Electrons in Atoms Powerpoint
... to the wavelength Different wavelengths will show different colors Each element gives off it own unique set of colors Therefore each element gives off its own unique amount of energy ...
... to the wavelength Different wavelengths will show different colors Each element gives off it own unique set of colors Therefore each element gives off its own unique amount of energy ...
R - University of St Andrews
... Transitions between any two Bohr energy states involve several spectral lines. This is known as fine structure. Explanation: each energy level actually consists of several distinct states with almost the same energy. The first theory that justified this was done by Wilson and Sommerfeld: they conjec ...
... Transitions between any two Bohr energy states involve several spectral lines. This is known as fine structure. Explanation: each energy level actually consists of several distinct states with almost the same energy. The first theory that justified this was done by Wilson and Sommerfeld: they conjec ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.