* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chem 150 Problem Set Introductory Quantum Chemistry 1
Survey
Document related concepts
Double-slit experiment wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Renormalization wikipedia , lookup
Particle in a box wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Tight binding wikipedia , lookup
Hydrogen atom wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Chemical bond wikipedia , lookup
Molecular orbital wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Wave–particle duality wikipedia , lookup
Atomic orbital wikipedia , lookup
Transcript
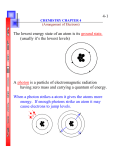
Chem 150 Problem Set Introductory Quantum Chemistry 1. Determine which of the following statements are false and correct them. a) Electromagnetic radiation is incapable of passing through water. b) Electromagnetic radiation travels through a vacuum at a constant speed, regardless of the wavelength. c) Infrared light has higher frequency than visible light. d) The glow from a fire place, the energy within a microwave oven and a for horn blast are all forms of electromagnetic radiation. 2. a) What is the frequency of radiation that has wavelength of 955 μm? b) What is the wavelength of radiation that has a frequency of 5.50 x 1014 s-1? c) Would the radiation in part a) or part b) be visible to the human eye? 3. Energy from radiation can be used to cause rupture in chemical bonds (wanted e.g. as in photosynthesis or in degradable plastics that decompose in sunlight or unwanted as in a sunburn). A minimum energy of 941 kJ/mol is required to break the nitrogen-nitrogen bond in N2. What is the longest wavelength of radiation that possesses the necessary energy to break the bond? What type of radiation is that? 4. It requires a photon with a minimum energy of 4.41 x 10 -19 J to emit electrons from sodium metal. a) What is the minimum frequency of radiation necessary to emit electrons from sodium metal? b) What is the wavelength of this light? 5. Calculate the de Broglie wavelength of a muon, a subatomic particle that decays within a few nanoseconds after formation traveling with a velocity of 8.85 x 10 5 cm s-1. The resting mass of a muon is 206.8 electron masses. 6. a) According to the Bohr model an electron in the ground state of a hydrogen atom orbits the nucleus at a specific radius of 0.53 x 10-10 m. In the quantum mechanical description of the hydrogen atom, the most probable distance of the electron from the nucleus is 0.53 x 10 -10 m. Why are these two statements different? b) Why is Schrődinger’s wave equation to describe the location of a particle very different from the description obtained from classical physics? c) In the quantum mechanical description of an electron what is the physical significance of the square of the wave function Ψ2? 7. Give the values for n, l, and ml for a) each orbital of the 2p subshell, b) each orbital in the 5 d subshell. 8. What is the maximum number of electrons in an atom that can have the following quantum numbers: a) n = 2, ms = -1/2; b) n = 5, l = 3; c) n = 4, l = 3, ml = -3; d) n = 4, l = 1, ml = 1. 9. a) What are valence electrons? b) What are unpaired electrons? c) How many valence electrons does a P atom possess and how many of them are unpaired? 10. Represent the electronic configuration of the following elements with an orbital diagram: F, S, Ar, Ti, and Ni. 11. Draw a 2pz orbital. Make sure to include a labelled axis or coordinate system on your drawing and show phasing either as + and – or shaded and not shaded. 12. What is the uncertainty in the position of an electron whose speed is known with an uncertainty of 1 m/s. 13. How does Valence bond theory describe the bonding in a) HCN and b) C2H4 (ethylene)? Sketch and label the atomic and/or hybrid orbitals that overlap. Clearly show phasing and occupancy of each orbital. How many σ-bonds and how many π-bonds (if any) have you formed in each molecule? 14. Given below is the molecular orbital diagram for diatomic species of the first period. Assume the species H2- was postulated to exist, a dihydrogen anion. a) How many electrons does this species possess? b) Show how the electrons occupy the molecular orbital diagram below. c) What is the bond order in H2- ? d) Would you expect a bond length longer or shorter as that in dihydrogen? e) Is the species dia- or paramagnetic? f) Should H2- exist? 15. Given below is an empty an unlabeled molecular orbital diagram of homonuclear diatomic species of the second period in the periodic table for atomic number Z equal or greater 8. a) Label the atomic as well as the molecular orbitals. b) How many of the molecular orbitals are bonding and how many are anti bonding? c) How does MO theory describe the bonding in fluorine gas? Fill the MO diagram with the appropriate number of electrons. What is the bond order in F2? d) Is F2 diamagnetic or paramagnetic? 16. Why does the conductivity of a semiconductor such as silicon increase with temperature? Formulas and constants pV = nRT piVi(Ti)-1 = pfVf(Tf)-1 Ptotal = p1 + p2 + p3 + .... + pn [p + n2a/V2][V – nb] = nRT Vmol = 22.4 L mol-1 at STP (1 atm = 101.325 kPa and 273.15 K) R = 8.3145 L kpa mol-1 K-1 Fundamental wave equation c = λν Plank’s equation E = hν Heisenberg’s uncertainty principle ΔxΔp ≥ h/4π De Broglie’s wave-particle dualism λ = h/p Impulse p = mv Kinetic energy Ekin = 0.5mv2 Planks constant h = 6.626 10-34 Js Avogadro’s number Na = 6.022 x 1023 mol-1 Unified atomic mass unit 1 u = 1.660 x 10-27 kg Mass of electron me = 9.109 x 10-31 kg Mass of proton mp = 1.673 x 10-27 kg Mass of neutron mn = 1.675 x 10-27 kg Speed of light c = 3 x 108 ms-1 End of formula sheet. The orbital pictures on the next page will not be on the formula sheet!