Chapter 2.2 and 7 Notes
... 5 orbitals, 2 electrons each 10 total electrons in each energy level Do not show up until the 3rd energy level ...
... 5 orbitals, 2 electrons each 10 total electrons in each energy level Do not show up until the 3rd energy level ...
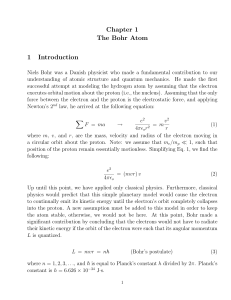
Chapter 1 The Bohr Atom 1 Introduction
... physics would predict that this simple planetary model would cause the electron to continually emit its kinetic energy until the electron’s orbit completely collapses into the proton. A new assumption must be added to this model in order to keep the atom stable, otherwise, we would not be here. At t ...
... physics would predict that this simple planetary model would cause the electron to continually emit its kinetic energy until the electron’s orbit completely collapses into the proton. A new assumption must be added to this model in order to keep the atom stable, otherwise, we would not be here. At t ...
Example 27-1
... •Electrons obit in stationary states that are characterized by a quantum number n and a discrete energy En. Sometimes this is called a energy level. •En is negative indicating a bound electron Z2 En 13.6 eV 2 n ...
... •Electrons obit in stationary states that are characterized by a quantum number n and a discrete energy En. Sometimes this is called a energy level. •En is negative indicating a bound electron Z2 En 13.6 eV 2 n ...
Quantum Theory and Electrons as Waves
... De Broglie proposed that electrons could behave like waves. ...
... De Broglie proposed that electrons could behave like waves. ...
Liad Elmelech 7.1-7.3 The Nature of Light, Atomic Spectroscopy
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
The Quantum Mechanical Behavior of Light and Matter
... classical: matter behaves like particles, light behaves like waves quantum mechanics: both matter and light behave like both particles and waves ...
... classical: matter behaves like particles, light behaves like waves quantum mechanics: both matter and light behave like both particles and waves ...
H-atom, emission spectra
... Electron loses energy to a photon - gives off light Electron goes to a lower energy level 13.6eV 13.6eV losing energy | E | nf 2 ni 2 ...
... Electron loses energy to a photon - gives off light Electron goes to a lower energy level 13.6eV 13.6eV losing energy | E | nf 2 ni 2 ...
Chapter 5 Review “Electrons in Atoms”
... orbital is “clockwise”, what is the spin of the other electron in that orbital? What is the approximate energy of a photon having a frequency of 4 x 107 Hz? (h = 6.6 x 10-34 J . s) Which of the following would be most stable: a) 4d55s1, or b) 4d45s2 ...
... orbital is “clockwise”, what is the spin of the other electron in that orbital? What is the approximate energy of a photon having a frequency of 4 x 107 Hz? (h = 6.6 x 10-34 J . s) Which of the following would be most stable: a) 4d55s1, or b) 4d45s2 ...
Chapter 5 Review “Electrons in Atoms”
... orbital is “clockwise”, what is the spin of the other electron in that orbital? What is the approximate energy of a photon having a frequency of 4 x 107 Hz? (h = 6.6 x 10-34 J . s) Which of the following would be most stable: a) 4d55s1, or b) 4d45s2 ...
... orbital is “clockwise”, what is the spin of the other electron in that orbital? What is the approximate energy of a photon having a frequency of 4 x 107 Hz? (h = 6.6 x 10-34 J . s) Which of the following would be most stable: a) 4d55s1, or b) 4d45s2 ...
1 - M*W
... a) Are the last column (to the right) on the periodic table b) Do not react with other elements c) Are the first column (on the left) on the periodic table d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They wa ...
... a) Are the last column (to the right) on the periodic table b) Do not react with other elements c) Are the first column (on the left) on the periodic table d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They wa ...
Spectrum of electron in quantum well with continuous position
... The rapid development of nanophysics stimulates the theoretical research of physical processes in multilayered heterostructures which are the elementary basis of modern nanodevices. For the deep understanding it is necessary do develop the adequate theory of quasiparticles states in nanoheterostruct ...
... The rapid development of nanophysics stimulates the theoretical research of physical processes in multilayered heterostructures which are the elementary basis of modern nanodevices. For the deep understanding it is necessary do develop the adequate theory of quasiparticles states in nanoheterostruct ...
Chapter 12 Worksheet
... a. the momentum of a particle cannot be measured precisely b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a particle cannot be measured precisely 4. From the follo ...
... a. the momentum of a particle cannot be measured precisely b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a particle cannot be measured precisely 4. From the follo ...
Degeneracy vs. Energy Level Scaling for Hydrogen
... where a0 = m~e e2 is the Bohr radius. Higher energy shells of Hydrogen are further away from the proton. Increasing the amount of orbiting electron charge by n2 would cancel this e↵ect and give the same potential energy for each shell. The total energy of the system is linear in the filling fraction ...
... where a0 = m~e e2 is the Bohr radius. Higher energy shells of Hydrogen are further away from the proton. Increasing the amount of orbiting electron charge by n2 would cancel this e↵ect and give the same potential energy for each shell. The total energy of the system is linear in the filling fraction ...
17-2 Example Problems Involving Potential Energy
... decreases as the distance between the balls increases. This makes it difficult to apply a force analysis. Energy conservation is a simpler approach. Our energy equation is: ...
... decreases as the distance between the balls increases. This makes it difficult to apply a force analysis. Energy conservation is a simpler approach. Our energy equation is: ...
Physical Chemistry The hydrogen atom Center of mass
... The spatial part is incomplete One incorporates spin as a separate coordinate Wave function is a product n ,l ,m , I ,mI (r , , ) ...
... The spatial part is incomplete One incorporates spin as a separate coordinate Wave function is a product n ,l ,m , I ,mI (r , , ) ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.