Chapter 5 PPT/Notes B
... • If single objects approach a double slit, they pass through and slowly build up, one by one, the double slit pattern. Shut one slit off and they, one by one, create the ...
... • If single objects approach a double slit, they pass through and slowly build up, one by one, the double slit pattern. Shut one slit off and they, one by one, create the ...
Glossary Chapter 4
... electromagnetic radiation a form of energy that exhibits wavelike behavior as it travels through space (91) electromagnetic spectrum all the forms of electromagnetic radiation (91) electron configuration the arrangement of electrons in an atom (105) excited state a state in which an atom has a highe ...
... electromagnetic radiation a form of energy that exhibits wavelike behavior as it travels through space (91) electromagnetic spectrum all the forms of electromagnetic radiation (91) electron configuration the arrangement of electrons in an atom (105) excited state a state in which an atom has a highe ...
Chapt25_VGO
... Sometimes electromagnetic waves (light) behave as if they were composed of particles (photons) In both cases an element of probability is introduced. We can no longer say what will happen in a set of circumstances, rather we can say what are the probabilities of various things ...
... Sometimes electromagnetic waves (light) behave as if they were composed of particles (photons) In both cases an element of probability is introduced. We can no longer say what will happen in a set of circumstances, rather we can say what are the probabilities of various things ...
Honors Chemistry
... orbital to fill after 3p would be 3d, but alas, it is not.. 4s is the next level we fill as it has lower energy than 3d. Look at potassium and calcium. Exceptions to the Aufbau Principle: Cr is ...
... orbital to fill after 3p would be 3d, but alas, it is not.. 4s is the next level we fill as it has lower energy than 3d. Look at potassium and calcium. Exceptions to the Aufbau Principle: Cr is ...
Spectroscopy - Birmingham City Schools
... c = speed of light (3 x 108 m/s) Since 1859 scientists using spectral lines to identify elements o Each element has characteristic spectral lines 1913 Bohr puts two and two together (spectra + quantum) o Remember Question… “Why don’t electrons fall into the nucleus?” o Bohr’s Answer e- hav ...
... c = speed of light (3 x 108 m/s) Since 1859 scientists using spectral lines to identify elements o Each element has characteristic spectral lines 1913 Bohr puts two and two together (spectra + quantum) o Remember Question… “Why don’t electrons fall into the nucleus?” o Bohr’s Answer e- hav ...
Chemistry I Honors – Semester Exam Review – Fall 2000
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m 4.84 10-19 J Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they ...
... a. 0.652 dm, b. 2,300 kg, c. 65 mL, d. 50,200 cm 1900 mL 8.7 hours slope = (mass) (volume) = density always record one estimate digit 1200 m 4.84 10-19 J Hydrogen atoms have specific energy levels. Therefore, the atoms can only gain or lose certain amounts of energy. When atoms lose energy, they ...
Chemistry—Chapter 13: Electrons in Atoms
... 3) What are the types of sublevels and number of orbitals in the following energy levels? a. n = 1 d. n = 4 b. n = 2 e. n = 5 c. n = 3 f. n = 6 II. Electron Arrangement in Atoms 4. Write a complete electron configuration of each atom, including orbital notations. Give the quantum numbers for the hig ...
... 3) What are the types of sublevels and number of orbitals in the following energy levels? a. n = 1 d. n = 4 b. n = 2 e. n = 5 c. n = 3 f. n = 6 II. Electron Arrangement in Atoms 4. Write a complete electron configuration of each atom, including orbital notations. Give the quantum numbers for the hig ...
Helium Atom
... from Helium atom = 24.63 eV When one electron is removed then the energy of ionized Helium ion (He+) = -Z2×13.6 eV =-54.4 eV So the ground state energy of Helium = -54.5 eV - 24.63 eV = -79.03 eV Thus, Atomic energies calculated in this manner are the poor approximation to the actual eigen ...
... from Helium atom = 24.63 eV When one electron is removed then the energy of ionized Helium ion (He+) = -Z2×13.6 eV =-54.4 eV So the ground state energy of Helium = -54.5 eV - 24.63 eV = -79.03 eV Thus, Atomic energies calculated in this manner are the poor approximation to the actual eigen ...
Chem Final Study Guide Energy How much heat energy must be
... 23) According to Niels Bohr's atomic model, what occurs when an atom absorbs radiated energy? a) The electron jumps to a higher energy level (excited state) 24) A hydrogen atom emits a photon of energy. Explain how this can happen. a) The electron jumped down to the ground state. 25) According to Bo ...
... 23) According to Niels Bohr's atomic model, what occurs when an atom absorbs radiated energy? a) The electron jumps to a higher energy level (excited state) 24) A hydrogen atom emits a photon of energy. Explain how this can happen. a) The electron jumped down to the ground state. 25) According to Bo ...
3. atomic structure
... in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to ide ...
... in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to ide ...
Wave Particle Duality Power Point NOTES
... which means they are QUANTIZED (quantity) wavelengths. ...
... which means they are QUANTIZED (quantity) wavelengths. ...
Modern Model of the Atom
... location of an electron like we can predict planets orbiting the sun! Different orbital shapes: s, p, d, f (lowest to highest energy) ...
... location of an electron like we can predict planets orbiting the sun! Different orbital shapes: s, p, d, f (lowest to highest energy) ...
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercury atom and anode current will decrease Coclusion: energy of Mercury atom cannot change continuously, but only by certain discrete values, so-called qua ...
... no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might become inelastic: electrons may transfer their energy to a Mercury atom and anode current will decrease Coclusion: energy of Mercury atom cannot change continuously, but only by certain discrete values, so-called qua ...
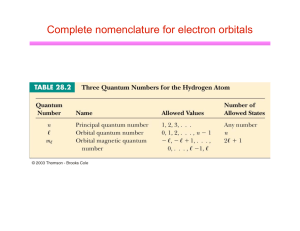
Complete nomenclature for electron orbitals
... Stimulated emission l Suppose an atom is in an excited state E2 and a photon with energy hf=E2-E1 is incident on the atom l Incoming photon of this energy increases the probability of the atom returning to the ground state emitting a photon of the same energy hf as the incident photon and in phase ...
... Stimulated emission l Suppose an atom is in an excited state E2 and a photon with energy hf=E2-E1 is incident on the atom l Incoming photon of this energy increases the probability of the atom returning to the ground state emitting a photon of the same energy hf as the incident photon and in phase ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.