Final Exam Review
... 44. Write the orbital diagram & electron configurations for the following elements: K, Ar, H, He, Br 45. Define precision and accuracy. 46. What determines an element’s order on the periodic table? 47. What happens to the temperature of a substance as it is changing states? 48. What is kinetic energ ...
... 44. Write the orbital diagram & electron configurations for the following elements: K, Ar, H, He, Br 45. Define precision and accuracy. 46. What determines an element’s order on the periodic table? 47. What happens to the temperature of a substance as it is changing states? 48. What is kinetic energ ...
Chapter 7 Handout 1 Atomic Orbitals Quantum Numbers: Principal
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
1 - theozone
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
1 - Revsworld
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
explo3
... Atomic and molecular physics: 2012 EXPLORATIONS –3 1) Sketch the energy level diagram of hydrogen atom, including the fine structure up to n=3. Show the possible transitions. How many different lines are there? ...
... Atomic and molecular physics: 2012 EXPLORATIONS –3 1) Sketch the energy level diagram of hydrogen atom, including the fine structure up to n=3. Show the possible transitions. How many different lines are there? ...
Electrons in Atoms
... • The line- (or atomic-) emission spectrum of an element is the set of frequencies of the EM waves emitted by atoms of the element. – Each element’s atomic emission spectrum is unique and can be used to determine if that element is present in a compound or to identify an element. ...
... • The line- (or atomic-) emission spectrum of an element is the set of frequencies of the EM waves emitted by atoms of the element. – Each element’s atomic emission spectrum is unique and can be used to determine if that element is present in a compound or to identify an element. ...
midterm answers
... the square of the wave function is the probability density, since the wave function is approaching zero without reaching it as long as x is finite, the square of the wave function will not reach zero either, this being the probability density in the barrier, the particle has a probability to be ther ...
... the square of the wave function is the probability density, since the wave function is approaching zero without reaching it as long as x is finite, the square of the wave function will not reach zero either, this being the probability density in the barrier, the particle has a probability to be ther ...
Exam #: _____________________ Printed Name: ________________ Signature:___________________ PHYSICS DEPARTMENT
... which are located at energy ED below the bottom of the continuum. The donor levels are far apart and do not interact whether or not they are occupied. a) What is the entropy of the nD electrons in the ND donor levels? Assume that the donor levels are each occupied by at most one electron, of either ...
... which are located at energy ED below the bottom of the continuum. The donor levels are far apart and do not interact whether or not they are occupied. a) What is the entropy of the nD electrons in the ND donor levels? Assume that the donor levels are each occupied by at most one electron, of either ...
Quanta3 - UF Physics
... quantized, such that En nhf , where h is a constant of nature. The emitted radiation is the difference in energy between two such energy levels: E hf . We will now see how Einstein made an even bolder proposal to explain the photoelectric effect. The photoelectric effect is the ejection of elec ...
... quantized, such that En nhf , where h is a constant of nature. The emitted radiation is the difference in energy between two such energy levels: E hf . We will now see how Einstein made an even bolder proposal to explain the photoelectric effect. The photoelectric effect is the ejection of elec ...
Ch4 notes - Midway ISD
... • Angular momentum quantum number (l) – indicates shape of orbital (sublevel) • l = zero and all positive integers less than or equal to n-1 • l=0, s orbital (spherical) • l=1, p orbital (dumbbell) • l=2, d orbital • l=3, f orbital ...
... • Angular momentum quantum number (l) – indicates shape of orbital (sublevel) • l = zero and all positive integers less than or equal to n-1 • l=0, s orbital (spherical) • l=1, p orbital (dumbbell) • l=2, d orbital • l=3, f orbital ...
P. LeClair
... 1. It takes 3×106 J of energy to fully recharge a 9 V battery. How many electrons must be moved across the ∆V = 9 V potential difference to fully recharge the battery? One electron has a charge of −e, given above. 2×1024 electrons. The energy required to charge the battery is just the amount that th ...
... 1. It takes 3×106 J of energy to fully recharge a 9 V battery. How many electrons must be moved across the ∆V = 9 V potential difference to fully recharge the battery? One electron has a charge of −e, given above. 2×1024 electrons. The energy required to charge the battery is just the amount that th ...
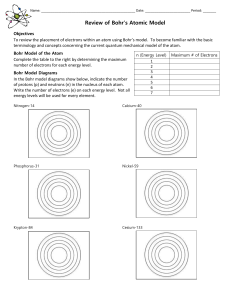
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... Review of Bohr’s Atomic Model Objectives ...
... Review of Bohr’s Atomic Model Objectives ...
ExamView - Untitled.tst
... ____ 13. Thomson made his discovery about the atom during an experiment using a. thermal energy. c. cathode rays b. kinetic energy. d. X rays. ____ 14. In _____ atomic model, negative electrons orbit the positively charged nucleus. a. Dalton’s c. Rutherford’s b. Thomson’s d. Democritus’s ____ 15. Wh ...
... ____ 13. Thomson made his discovery about the atom during an experiment using a. thermal energy. c. cathode rays b. kinetic energy. d. X rays. ____ 14. In _____ atomic model, negative electrons orbit the positively charged nucleus. a. Dalton’s c. Rutherford’s b. Thomson’s d. Democritus’s ____ 15. Wh ...
Degeneracy of Hydrogen atom
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
Pre-AP Chemistry
... 10. What type of spectra would be created by exciting xenon gas? 11. What element did Bohr use to support his theory on bright line spectra? 12. Energy of a photon is directly proportional to what wave property? 13. What is a spectrum? 14. What two forms of energy can generally be used to excite an ...
... 10. What type of spectra would be created by exciting xenon gas? 11. What element did Bohr use to support his theory on bright line spectra? 12. Energy of a photon is directly proportional to what wave property? 13. What is a spectrum? 14. What two forms of energy can generally be used to excite an ...
You may recall the formula: V = W/q Potential difference between
... 1) Yellow light with a frequency of 5.43 x 1014 Hz strikes a cesium surface. If the photoelectric work function of cesium is 3.42 x 1019 J, what is the maximum velocity a photoelectron emitted from the surface can have? (mass of electron = 9.11 x 1031 kg) hf − φ = ½ mv2 (6.626x10-34 Js)(5.4 ...
... 1) Yellow light with a frequency of 5.43 x 1014 Hz strikes a cesium surface. If the photoelectric work function of cesium is 3.42 x 1019 J, what is the maximum velocity a photoelectron emitted from the surface can have? (mass of electron = 9.11 x 1031 kg) hf − φ = ½ mv2 (6.626x10-34 Js)(5.4 ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.