Basic Chemistry Notes II
... 3. The atomic number is the number of protons B. Neutrons 1. Found in nucleus 2. No charge 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most impo ...
... 3. The atomic number is the number of protons B. Neutrons 1. Found in nucleus 2. No charge 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most impo ...
The Quantum Theory of Atoms and Molecules
... 1. It fails to provide any understanding of why certain spectral lines are brighter than others. There is no mechanism for the calculation of transition probabilities. ...
... 1. It fails to provide any understanding of why certain spectral lines are brighter than others. There is no mechanism for the calculation of transition probabilities. ...
3. Analysis of distribution functions
... Using lecture-notes and referenced literature [1, p. 38–52], examine principles of statistical physics, distribution functions and properties of electrons in metals and semiconductors. Prepare to answer the questions: What statistics can by applied to electrons in a metal? What statistics is applied ...
... Using lecture-notes and referenced literature [1, p. 38–52], examine principles of statistical physics, distribution functions and properties of electrons in metals and semiconductors. Prepare to answer the questions: What statistics can by applied to electrons in a metal? What statistics is applied ...
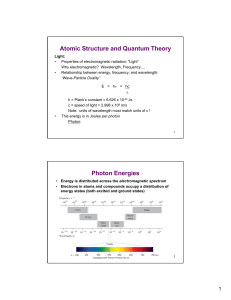
Spectroscopy
... marked in minutes of arc. One degree is 60 arc-minutes; one minute of arc is 60 arc-seconds. Thus the vernier scale shows 0-30 minutes of half a degree. Of course, when using the equation above, the angle must be converted to decimal fractions of a degree. Also, the etched lines are not filled in wi ...
... marked in minutes of arc. One degree is 60 arc-minutes; one minute of arc is 60 arc-seconds. Thus the vernier scale shows 0-30 minutes of half a degree. Of course, when using the equation above, the angle must be converted to decimal fractions of a degree. Also, the etched lines are not filled in wi ...
Seeing Atoms and Electrons in Motion - The Munich
... However, our world is not static. Any reaction or process is essentially defined by movement paths on a (sub-)atomic level. ...
... However, our world is not static. Any reaction or process is essentially defined by movement paths on a (sub-)atomic level. ...
File
... Electronegativity – ability of an atom to attract an electron TIME AMOUNT Nuclear Chemistry ...
... Electronegativity – ability of an atom to attract an electron TIME AMOUNT Nuclear Chemistry ...
Midterm Exam 2
... Oxygen and nitrogen are two of the major components of the atmosphere as well as critical components for all biological system. Both elements exist as diatomic gases (X2) within the atmosphere. O2 is a reactive species which is often involved in oxidation. This process leads to the formation or rust ...
... Oxygen and nitrogen are two of the major components of the atmosphere as well as critical components for all biological system. Both elements exist as diatomic gases (X2) within the atmosphere. O2 is a reactive species which is often involved in oxidation. This process leads to the formation or rust ...
Chpater 5.3 PPT
... Usually attracted more to one atom This will effect the chemical properties of the ...
... Usually attracted more to one atom This will effect the chemical properties of the ...
Chapter 7
... Follow 3 rules to configure the electrons 1. Aufbau Principle - electrons fill orbitals starting at the lowest available (possible) energy states before filling higher states 2. Pauli Exclusion Principle - two electrons cannot share the same set of quantum numbers within the same system. Therefore, ...
... Follow 3 rules to configure the electrons 1. Aufbau Principle - electrons fill orbitals starting at the lowest available (possible) energy states before filling higher states 2. Pauli Exclusion Principle - two electrons cannot share the same set of quantum numbers within the same system. Therefore, ...
Energy and Matter - Hicksville Public Schools
... configurations are prescribed by three rules: the aufbau principle – states that in the ground state electrons occupy the lowest energy orbitals available, the Pauli exclusion principle – states that only up to 2 electrons can occupy an orbital, and the Hund’s rule – describes how electrons fill orb ...
... configurations are prescribed by three rules: the aufbau principle – states that in the ground state electrons occupy the lowest energy orbitals available, the Pauli exclusion principle – states that only up to 2 electrons can occupy an orbital, and the Hund’s rule – describes how electrons fill orb ...
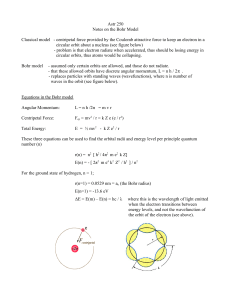
Astr 250 Notes on the Bohr Model Classical model
... These three equations can be used to find the orbital radii and energy level per principle quantum number (n) r(n) = n2 [ h2 / 4π2 m e2 k Z] E(n) = - [ 2π2 m e4 k2 Z2 / h2 ] / n2 For the ground state of hydrogen, n = 1; r(n=1) = 0.0529 nm = ao (the Bohr radius) E(n=1) = -13.6 eV ΔE = E(m) – E(n) = h ...
... These three equations can be used to find the orbital radii and energy level per principle quantum number (n) r(n) = n2 [ h2 / 4π2 m e2 k Z] E(n) = - [ 2π2 m e4 k2 Z2 / h2 ] / n2 For the ground state of hydrogen, n = 1; r(n=1) = 0.0529 nm = ao (the Bohr radius) E(n=1) = -13.6 eV ΔE = E(m) – E(n) = h ...
Definitions are in Book
... 2) How are Hess’s law, ∆Hof, and the fact that enthalpy is a state function all connected? As discussed in the SI sessions and the test review, enthalpy is a state function—meaning it doesn’t matter how you get from the starting point to the end point, the change is always the same. We can think of ...
... 2) How are Hess’s law, ∆Hof, and the fact that enthalpy is a state function all connected? As discussed in the SI sessions and the test review, enthalpy is a state function—meaning it doesn’t matter how you get from the starting point to the end point, the change is always the same. We can think of ...
Honors Chemistry
... Assign electron configurations to atoms and ions. Assign quantum numbers to electrons. Identify core and valence electrons using the periodic table. Define and apply orbital filling rules. ...
... Assign electron configurations to atoms and ions. Assign quantum numbers to electrons. Identify core and valence electrons using the periodic table. Define and apply orbital filling rules. ...
Exam #2
... Ethanol (molar mass = 46.05 g/mol), C2H5OH, is mixed with gasoline and sold as gasohol. Use the following to calculate the grams of ethanol needed to provide 293 kJ of heat: ...
... Ethanol (molar mass = 46.05 g/mol), C2H5OH, is mixed with gasoline and sold as gasohol. Use the following to calculate the grams of ethanol needed to provide 293 kJ of heat: ...
PHYSICS 215 - Thermodynamics and Modern Physics Name:
... Speed of light, c = 3.00E8 m/s Charge of an electron, -e = -1.6E-19 C Mass of the electron, me = 9.1E-31 kg = 511 keV/c2 = 5.49E-4 u Mass of the proton, mp = 1.67E-27 kg = 938 MeV/c2 = 1.00728 u Mass of the α particle, mα = 3727.4 MeV/c2 = 4.00151 u ...
... Speed of light, c = 3.00E8 m/s Charge of an electron, -e = -1.6E-19 C Mass of the electron, me = 9.1E-31 kg = 511 keV/c2 = 5.49E-4 u Mass of the proton, mp = 1.67E-27 kg = 938 MeV/c2 = 1.00728 u Mass of the α particle, mα = 3727.4 MeV/c2 = 4.00151 u ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.