Transcript - the Cassiopeia Project
... An electron in the lowest energy shell in an atom can be struck by and absorb the energy of a photon giving it enough energy to jump to the next energy shell. And the reverse process allows the electron to jump back down into the lowest energy shell and emit a photon. The color of the photon depends ...
... An electron in the lowest energy shell in an atom can be struck by and absorb the energy of a photon giving it enough energy to jump to the next energy shell. And the reverse process allows the electron to jump back down into the lowest energy shell and emit a photon. The color of the photon depends ...
Moderne Methoden der Materialcharakterisierung
... Cathode material determines emission current density ...
... Cathode material determines emission current density ...
Energy Expectation Values and the Origin of the Variation Principle
... statistically meaningful number of such states are available for the purpose of measuring the energy. Quantum mechanical principles state that an energy measurement must yield one of the energy eigenvalues, ,i, of the energy operator. Therefore, the average value of the energy measurements is calcul ...
... statistically meaningful number of such states are available for the purpose of measuring the energy. Quantum mechanical principles state that an energy measurement must yield one of the energy eigenvalues, ,i, of the energy operator. Therefore, the average value of the energy measurements is calcul ...
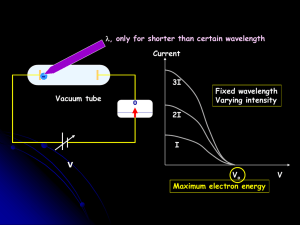
The Photoelectric Effect, work function
... 1. A negatively charged zinc plate lost its charge when subjected to UV light. implications? Electrons must be leaving the plate and going into the air/vacuum. These electrons will be called photoelectrons. 2. Light of a lower frequency (e.g. red light) has no effect.(recall: lower frequency = lo ...
... 1. A negatively charged zinc plate lost its charge when subjected to UV light. implications? Electrons must be leaving the plate and going into the air/vacuum. These electrons will be called photoelectrons. 2. Light of a lower frequency (e.g. red light) has no effect.(recall: lower frequency = lo ...
l3_bondingebands
... Wave momentum k only unique up to 2π/a Only certain electron energies allowed, but those can propagate (theoretically) unimpeded, as long as lattice spacing is “perfectly” maintained But, resistance introduced by: ________ and ________ ...
... Wave momentum k only unique up to 2π/a Only certain electron energies allowed, but those can propagate (theoretically) unimpeded, as long as lattice spacing is “perfectly” maintained But, resistance introduced by: ________ and ________ ...
homework answers - SPHS Devil Physics
... a. Describe emission and absorption spectra and understand their significance for atomic structure b. Explain the origin of atomic energy levels in terms of the ‘electron in a box’ model c. Describe the hydrogen atom according to Schrödinger d. Do calculations involving wavelengths of spectral lines ...
... a. Describe emission and absorption spectra and understand their significance for atomic structure b. Explain the origin of atomic energy levels in terms of the ‘electron in a box’ model c. Describe the hydrogen atom according to Schrödinger d. Do calculations involving wavelengths of spectral lines ...
Chemistry 2000 Review: quantum mechanics of
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.