* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ground State
Multi-state modeling of biomolecules wikipedia , lookup
History of chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atomic nucleus wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Pseudo Jahn–Teller effect wikipedia , lookup
Photoelectric effect wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical bond wikipedia , lookup
Atomic orbital wikipedia , lookup
History of molecular theory wikipedia , lookup
Hydrogen atom wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Marcus theory wikipedia , lookup
Spin crossover wikipedia , lookup
Franck–Condon principle wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Metallic bonding wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Condensed matter physics wikipedia , lookup
Computational chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Heat transfer physics wikipedia , lookup
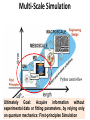
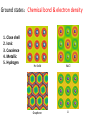
Introduction to Computational Chemistry and Materials 武晓君 (Xiaojun Wu) [email protected] 江俊 (Jun Jiang) [email protected] Question 1:Why Model and Simulation? Conventional style for making new material Try Sample Metallic Wire Error 3 Years Plant/Carbon Wire Question 1:Why Model and Simulation? After 130 years New Style for New Material Experiment Theory Simulation photon nucleus electron Material Characterization at the atomic scale TEM transmission electron microscope Spring with Carbon Nanotube Life Science Macroscopic view Cell View Single-molecue View Chemical Reaction Conventional reaction State-State reaction Single-molecule reaction Single Atom/Molecule Manipulation In Touch with Atoms • AFM,STM,SPM In Touch with Atoms Quantum Corral Controlled bond formation at the spatial limit. Science 286,1719(1999) Growing Importance of Computational Modeling Nuclear Reaction Safeguarding the Nuclear Stockpile through Computer Simulation Supercomputers study reactor core behaviour http://www.world-nuclear-news.org/nn-supercomputers_study_reactor_core_behaviour-2701104.html Growing Importance of Computational Modeling Calculating things that are difficult to do experimentally: Inside the Earth The melting curve of Iron at the pressures of the Earth’s core from ab initio calculations. Source: http://chianti.geol.ucl.ac.uk/~dario/resint.htm Alfe’ et al , Nature 401, 462, (1999) Growing Importance of Computational Modeling First Principles Materials Modeling in Industry Materials Genome Motorola IBM Dynamics of vortices Large-scale simulations of human bone under elastic deformations Siemens Eastman Kodak Phillips Xerox Electricité de France Texas Instruments Ford Nippon Steel Matsushita (Panasonic) Ricoh Allied Signal Alcoa Toyota General Motors TDK Lucent Hitachi Corning http://www.zurich.ibm.com/mcs/compsci/engineering/vortices.html Engine turbine Metal Extreme Conditions: 3000K,10GPa Engine turbine Metal Metal melting 70,000 atoms(Al, Fe, Cu) Engine turbine Metal Metal melting : 70,000 atoms(Al, Fe, Cu) Engine turbine Metal Save: 43, 200, 000 RMB Simulation cost: 200,000 RMB Question 1:Why Model and Simulation? Answer • Understand complex systems, processes, and phenomena at the fundamental and quantitative level • Bridge theory and experiment • Wide applications in Physics, Chemistry, Material, Life, Information science What is Model and Simulation? Model? Simplified Description of Actual System Nuclei and electron (electronic-structure level) Atoms and molecules (atomistic or molecular) Coupled structural elements (finite-element) Medium described by fields and distributions (continuum) Cu Crystal: potential U(r1, r2, …, rN) various properties How? Process to do this is Simulation What is useful and key to a good model? • The complex system can be simplified to – Base structure Clear – Interactions among particles Clear Key 1: What kind of models are employed to simplify the systems? Key 2: What are the specific problems of the realistic applications? Key 3: What are the limits of the models and methods? Multi-Scale Simulation Ultimately Goal: Acquire information without experimental data or fitting parameters, by relying only on quantum mechanics: First-principles Simulation QM Simulation Start with quantum mechanics and electronic structure Modern physics and chemistry applications rely on the detection and manipulation of electron kinetics: the space, time, energy information of electrons. Energy transfer Photon/ Electron Electronic(excited) states generation and coupling Material Conversion Movement and evolution c.b. e H2O O2 Semicond h+ v.b. H+ CO2 H2 Gas Photocatalysis Mol-Photonics Mol-Electronics Optoelectronics Catalysis Photo-detector Electronic structure decides material property 1890s Electron is a particle 1896 Pieter Zeeman, Lorentz “spectra line splitting” in magnetic filed 1902 Nobel Prize 1897 J J Thomson “Find electron in experiment “ negative charged 1906 Nobel Prize 1901 Rutherford 1911 Bohr “Find positive charged nuclei” experimentally Quantum Mechanics “discrete set of allowed levels of electrons” De Brogil,Schrodinger, Heisenberg Electron has spin 1925 Periodic Tablet 1926 Pauli exclusion theory “No two electrons at same Quantum State” 1927 Fermi Bose-Einstein Statistics Electron Electronic Structure : Molecular Lewis 1916 JACS, 38, 762 “The atom and the molecule” Electrons are delocalized over atoms forming “Chemical Bond” Electronic Structure : Condensed Matter A system made of many particles (atoms, molecules, ions, …) which interacting strongly with each other: Solid, Liquid, … …. In principle: Many-body problem Independent Particle Approximation +: Fermi – Dirac Distribution - : Bose-Einstein Distribution Occupation Number Energy of Independent particle Electronic Structure : Solid Independent electron + periodic potential of crystal lattice Wavefunction in solid is the eigenstate of “Crystal Momentum” Electronic Band Metal: Electrons move freely Insulator (Ionic , Valence,Molecular) Semiconductor Band Theory Electronic Structure of Diamond G.E.Kimball, J. Chem. Phys. 3:560, 1935 The first quantitative calculations of electronic states for Na metal The firs quantitatively accurate calculations of bands in semiconductor Ge Beyond the Independent Particle Electron Correlation (Challenge) What are results of electron correlation: 1. Magnetism: Exchange energy of interacting electrons 2. Metal-Insulator Transition: Many-body effects (1950s) Developments: • Advanced quantum chemistry method • Density functional theory method • Quantum Monte Carlo • Dynamic Mean-field Theory • Many-body perturbation What we want to Know? Electronic structure of ground and excited states Ground State – – – – – – – – Cohesive energy Crystal Structure Phase transition Elastic constant Charge density Magnetic property Dielectric Nuclear vibration and motion – Metal/Semiconductor/Insul ator – …… Excited State – Low energy excitations – Specific heat in metal – Transport – Pauli spin susceptibility – High energy excitations – Gap – Optical properties – Spectra for adding or removing electrons – …… Ground states:Chemical bond & electron density 1. 2. 3. 4. 5. Close shell Ionic Covalence Metallic Hydrogen He Solid NaCl Graphene Li Electron density:atomic charge distribution Si deformation charge density Experimental measurement: scattering of X-ray or High-energy electrons Ground state:Volume & Pressure Stable / equilibrium structure with volumn (Ω) under certain pressure (P) and temperature (T) decide material properties -14.2 2 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 3 -14.4 -14.6 -14.8 -15 Series1 -15.2 -15.4 -15.6 -15.8 -16 Diamond E .vs. lattice Bulk modulus of 3d/4d transition metal Pressure/stress induced phase transition Free Energy Gibbs free energy Enthalpy H Phase Transition Pressure/Stress 8GP Energy vs. Volume “an impressive achievement of “firstprinciples” theory with no adjustable parameters? Lowest transition pressure for tetrahedral semiconductors to a high-pressure phase Ground state:Elasticity Stress tensor Strain Isotropic material Young’s Modulus Poisson’s ratio Bulk modulus Shear modulus Ground state:Magnetism Many-body effect caused by electron-electron interaction Open shell atoms Hund’s rule: the ground state has the maximum total spin and maximum orbital moment Zeeman field Hzeeman = m(r) Vm(r) exchange-correlation energy Total Energy Magnetic susceptibility Spin Charge Density Distribution Graphene Piece Ground state:Vibrational & phonon Vibrational Spectra Experimental: infrared absorption Light scattering inelastic neutron scattering other… RI FI CIJ position of nuclei I Force on nuclei I Force constant Usually, difference within experiments ~ 5% Two methods: 1. “Frozen phonon” direct calculation energy with atomic positions 2. “Response Function” calculation of derivations of energy explicitly at any order Atom kinetics:diffusion, reaction, catalysis Linear Synchronous Transit (LST) Quadratic Synchronous Transit (QST) Nedged-Elastic Band (NEB) (Henkelman, G.; Jonsson, H. "Improved tangent estimate in the nudged elastic band method for finding energy paths and saddle points", J. Chem. Phys., 113, 9978 (2000).) Surface, Interface Surface energy Approximation: 1. T = 0, E(AB) chose the crystal energy 2. Chemical potential: U(A)+U(B)= U(AB) = E(AB) 3. The range of U, U(A)<= E(A) (bulk) , U(B) <= E(B) Excited states: band gap Excited state with same number electrons as ground state specific heat, line response, optical properties N -> N+1, or N -> N-1 photoemission, or inverse photoemission Fundamental Gap E gap min =min{(E[n+1]-E[n]) – (E[n]-E[n-1])} Photoemssion Spectroscopy (PES) Au (111) Excited state: heat, conductivity… • Keep electron number Electron-Hole interaction (EHI) E ex min < E gap min LDA underestimate the gap GW with (dot) and without (dash line) EHI Overview-I Overview-II Software • Free Siesta, Abinit, PWscf … • Non-free Gaussian, DMol3, CASTEP, VASP, Wien2K …