Chapter 4 - Rothschild Science
... Scientists started doing a lot of experiments looking at the absorption and emission of light by matter. Found that there is a relationship between light and an atom’s electrons. ...
... Scientists started doing a lot of experiments looking at the absorption and emission of light by matter. Found that there is a relationship between light and an atom’s electrons. ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
Matter and Energy Identify a chemical physical change Identify a
... Aufbau Principle Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
... Aufbau Principle Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
... I 10. (1 0) The decay between two excited states of the nucle~isof 4 ' ~ iemits gamma ray of 1.3117 MeV. Tht luppe, state has a lifetime of 1.4ps, the lower state 3.0 ps. A) What is the fractional uncertainty AEIE in tht energy of the gainma ray? B) What is the percentage spread in wavelength of the ...
Quantum Mechanics and the Bohr Model - slater science
... • Using the line as the midpoint draw two waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
... • Using the line as the midpoint draw two waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
Sugárkémiai áttekintés Schiller Róbert
... Delementary A 2 r One must know the activity of the source, then Delementary must be integrated over source and irradiated space. ...
... Delementary A 2 r One must know the activity of the source, then Delementary must be integrated over source and irradiated space. ...
Spectra of Atoms
... m c a0 (5 105 eV / c 2 )(0.53 A) e2a 2 (1.6 10 19 C ) 2 (9 10 22 m / s 2 ) 2 (9 109 Jm / C 2 ) ...
... m c a0 (5 105 eV / c 2 )(0.53 A) e2a 2 (1.6 10 19 C ) 2 (9 10 22 m / s 2 ) 2 (9 109 Jm / C 2 ) ...
Periodic Trends
... First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
... First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
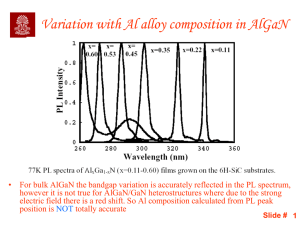
Slide 1
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
Inertia and E = Mc2
... and since e2/2(ct) is a measure of the electric field energy outside the radius ct, t being time, that remains to be accelerated as the disturbance progresses at speed c, it is evident that here we have a formula that tells us that an electron will accelerate in just such a way as to avoid shedding ...
... and since e2/2(ct) is a measure of the electric field energy outside the radius ct, t being time, that remains to be accelerated as the disturbance progresses at speed c, it is evident that here we have a formula that tells us that an electron will accelerate in just such a way as to avoid shedding ...
Mark scheme for Extension Worksheet – Topic 6, Worksheet 2
... electrons but the current only depends on the number of electrons emitted per second not their speed. ...
... electrons but the current only depends on the number of electrons emitted per second not their speed. ...
photoelectric effect
... directly proportional to the square of the electric field of the wave. An electron in some material exposed to this light wave should feel a force proportional to this electric field. For an intense enough illuminating light, the electron should be able to gain sufficient kinetic energy to escape th ...
... directly proportional to the square of the electric field of the wave. An electron in some material exposed to this light wave should feel a force proportional to this electric field. For an intense enough illuminating light, the electron should be able to gain sufficient kinetic energy to escape th ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.