
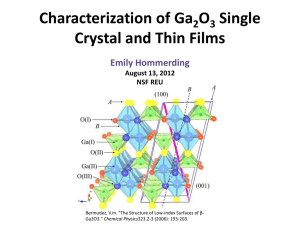
Scanning Electron Microscopy / Electron Probe X
... contrast. Within each layer, many crystals can be discerned due to the sensitivity of BSE’s for crystal orientation. ...
... contrast. Within each layer, many crystals can be discerned due to the sensitivity of BSE’s for crystal orientation. ...
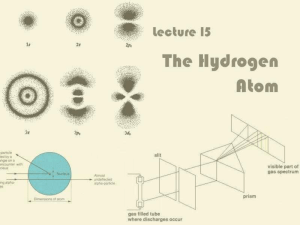
Ch. 5.1 Models of the Atom
... an electron can have, but also describes the probability of finding an electron at various locations around the nucleus, called atomic orbitals. Energy levels are labeled by principal quantum numbers (n). n = 1, 2, 3, etc. Several orbitals with different shapes and energy levels (sublevels) exist wi ...
... an electron can have, but also describes the probability of finding an electron at various locations around the nucleus, called atomic orbitals. Energy levels are labeled by principal quantum numbers (n). n = 1, 2, 3, etc. Several orbitals with different shapes and energy levels (sublevels) exist wi ...
Modern Physics - Leaving Cert Physics
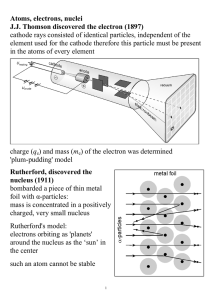
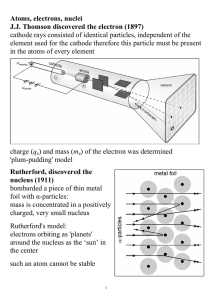
... filament which in turn heats the cathode. 2. Electrons are emitted from the hot cathode due to Thermionic Emission. 3. They get accelerated across the vacuum due to the very high voltage and smash into the high-density anode (usually tungsten) . 4. Most of the kinetic energy gets converted to heat, ...
... filament which in turn heats the cathode. 2. Electrons are emitted from the hot cathode due to Thermionic Emission. 3. They get accelerated across the vacuum due to the very high voltage and smash into the high-density anode (usually tungsten) . 4. Most of the kinetic energy gets converted to heat, ...
(n=1).
... En= -13.6 Z2/n2 • Photon emitted when electron jumps from high energy to low energy orbit. Photon absorbed when electron jumps from low energy to high energy: | E1 – E2 | = h f = h c / l ...
... En= -13.6 Z2/n2 • Photon emitted when electron jumps from high energy to low energy orbit. Photon absorbed when electron jumps from low energy to high energy: | E1 – E2 | = h f = h c / l ...
Skill Assessment Sheet Modern Atomic Theory (Quantum Mechanics)
... 1- Below Basic: 2- Basic: 3- Proficient: 4- Advanced: ...
... 1- Below Basic: 2- Basic: 3- Proficient: 4- Advanced: ...
The study of biology can help you better understand
... How many energy sublevels are contained in each of the hydrogen atom’s first three energy levels? (5.2) ...
... How many energy sublevels are contained in each of the hydrogen atom’s first three energy levels? (5.2) ...
Review 1st Qtr KEY
... 2. A spherical electron cloud surrounding an atomic nucleus would best represent a. an s orbital. c. a combination of px and py orbitals. b. a px orbital. d. a combination of an s and a px orbital. ...
... 2. A spherical electron cloud surrounding an atomic nucleus would best represent a. an s orbital. c. a combination of px and py orbitals. b. a px orbital. d. a combination of an s and a px orbital. ...
topic-2.doc
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
Chemistry Name______________________________________
... occupy one at a time. They will gain energy to jump to higher orbit and lose energy to fall to lower lowest energy for atom (all electrons in orbits closest to nucleus) couldnot explain other atom’s spectra ...
... occupy one at a time. They will gain energy to jump to higher orbit and lose energy to fall to lower lowest energy for atom (all electrons in orbits closest to nucleus) couldnot explain other atom’s spectra ...
Chapter2. Elements of quantum mechanics
... A photoelectric experiment indicates that violet light of wavelength 420 nm is the longest wavelength radiation that can cause photoemission of electrons from a particular multialkali photocathode surface. a. What is the work function of the photocathode surface, in eV? b. If a UV radiation of wavel ...
... A photoelectric experiment indicates that violet light of wavelength 420 nm is the longest wavelength radiation that can cause photoemission of electrons from a particular multialkali photocathode surface. a. What is the work function of the photocathode surface, in eV? b. If a UV radiation of wavel ...
3 — Blackbody Radiation [Revision : 1.5]
... First factor is volume of k-space octant between (k, k + dk); second factor is number of standing waves in volume of k space (extra 2 due to two possible polarization directions) ...
... First factor is volume of k-space octant between (k, k + dk); second factor is number of standing waves in volume of k space (extra 2 due to two possible polarization directions) ...
final2012
... b) If you use a photon of wavelength 100nm to ionize the electron in this system what is the stopping potential (in V) for the ionized electron. (15 points) 2) A gold foil is used in a Rutherford experiment to scatter alpha particles with energies 10 MeV. What fraction of the particles are backscatt ...
... b) If you use a photon of wavelength 100nm to ionize the electron in this system what is the stopping potential (in V) for the ionized electron. (15 points) 2) A gold foil is used in a Rutherford experiment to scatter alpha particles with energies 10 MeV. What fraction of the particles are backscatt ...
(s) If 5.00 moles of zinc is placed into 1.50 L... 34. solution,what is the mass of the hydrogen gas produced?
... Zn (s) + 2 HCI (aq) ~ Znc~ (s) +Hz(g) If 5.00 moles of zinc is placed into 1.50 L of a 3.00MHCI 34. solution,what is the mass of the hydrogen gas produced? (A) 0.750 g (D) 5.00 g (B) 2.25 g (E) 10.0 g (C) 4.50 g 32. How many grams of Zinc (atomic mass 65.0 g) are required to react completelywith 1.0 ...
... Zn (s) + 2 HCI (aq) ~ Znc~ (s) +Hz(g) If 5.00 moles of zinc is placed into 1.50 L of a 3.00MHCI 34. solution,what is the mass of the hydrogen gas produced? (A) 0.750 g (D) 5.00 g (B) 2.25 g (E) 10.0 g (C) 4.50 g 32. How many grams of Zinc (atomic mass 65.0 g) are required to react completelywith 1.0 ...
Example solution to the exercise 1
... Find a way to express the total energy as a function of the radius r. After this you can use the Larmor equation and the chain rule df (x) df (x) dx ...
... Find a way to express the total energy as a function of the radius r. After this you can use the Larmor equation and the chain rule df (x) df (x) dx ...
Chapter 5 Electrons In Atoms 5.1 Models of the Atom The
... Each energy sublevel corresponds to an orbital of a different shape, which describes where the _____________________ is likely to be found. Four of the five kinds of d orbitals have clover leaf shapes. The lowest principal energy level (n=1) has only one sublevel, called___________ The number of el ...
... Each energy sublevel corresponds to an orbital of a different shape, which describes where the _____________________ is likely to be found. Four of the five kinds of d orbitals have clover leaf shapes. The lowest principal energy level (n=1) has only one sublevel, called___________ The number of el ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? E= nhc/λ; where E is the energy per minute and n is the number of photons per minute n= E λ/(hc); n =[ (.200J/60)*(300 x 10-9m)]/[(6.626 x 10-34J s)(3.0 x 108 ms-1)] = 5.0 x 1015 15. A photon with some ...
... 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? E= nhc/λ; where E is the energy per minute and n is the number of photons per minute n= E λ/(hc); n =[ (.200J/60)*(300 x 10-9m)]/[(6.626 x 10-34J s)(3.0 x 108 ms-1)] = 5.0 x 1015 15. A photon with some ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.







![3 — Blackbody Radiation [Revision : 1.5]](http://s1.studyres.com/store/data/005908504_1-5005bdffc2e5f9c6e0687c31f49c7e9d-300x300.png)








