Modern physics 2330
... 3- ( ) The photoelectric effect takes place only if the energy of the incident electrons exceeds the energy of the photons. 4- ( ) X-rays are produced by bombarding a metal target with energetic electrons having energies of 50 to 100 KeV. 5- (..) A black hole is an object of sufficiently high densit ...
... 3- ( ) The photoelectric effect takes place only if the energy of the incident electrons exceeds the energy of the photons. 4- ( ) X-rays are produced by bombarding a metal target with energetic electrons having energies of 50 to 100 KeV. 5- (..) A black hole is an object of sufficiently high densit ...
1. Millikan did his experiments with the balance of
... repeating this experiment several times, he found that the values measured are always multiples of the same number. He then interpreted that this number is the charge of an electron: 1602 × 10-19 coulomb (SI unit for electric charge). ...
... repeating this experiment several times, he found that the values measured are always multiples of the same number. He then interpreted that this number is the charge of an electron: 1602 × 10-19 coulomb (SI unit for electric charge). ...
Electronic Structure and the Periodic Table A. Bohr Model of the
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
Quantum Mechanical Model of the Atom and Electronic Structure 1
... For each principal energy level designated by the principal quantum number n (n = 1, 2, 3, ...) there are n sublevels. The sublevels are characterized by the angular momentum quantum number l and are designated s, p, d, f, g ... For a given value of n, sublevel energy varies s < p < d < f ... ...
... For each principal energy level designated by the principal quantum number n (n = 1, 2, 3, ...) there are n sublevels. The sublevels are characterized by the angular momentum quantum number l and are designated s, p, d, f, g ... For a given value of n, sublevel energy varies s < p < d < f ... ...
Chemistry 1 Concept 5 “Electrons in Atoms” Study Guide
... 2. Specific wavelengths of light seen through a prism that are made when high voltage current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, ...
... 2. Specific wavelengths of light seen through a prism that are made when high voltage current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, ...
Exam sample
... 7. “No two electrons in the same atom may have the same values for all four quantum numbers” is a statement of: a. Hund’s Rule. b. deBroglie’s Hypothesis. c. the Pauli Exclusion Principle. d. the Heisenberg Uncertainty Principle. 8. All s orbitals are: a. shaped like four-leaf clovers. b. dumbbell- ...
... 7. “No two electrons in the same atom may have the same values for all four quantum numbers” is a statement of: a. Hund’s Rule. b. deBroglie’s Hypothesis. c. the Pauli Exclusion Principle. d. the Heisenberg Uncertainty Principle. 8. All s orbitals are: a. shaped like four-leaf clovers. b. dumbbell- ...
CHM 50- Class activity
... Complete the table of wavelength, frequency and energy values. Wavelength Frequency Energy per photon ...
... Complete the table of wavelength, frequency and energy values. Wavelength Frequency Energy per photon ...
November 18
... and how much energy does it have? To find frequency, use first formula, c=lambda x frequency Frequency = 3x 108 m/s / 700 x 10-9 meters = 4.29 x 1014 Hz To find Energy, plug it into the second formula: E = hf = (6.6 x 10-34) x (4.29 x 1014 Hz) = 2.82 x 10-19 Joules What is Fire? Excited electrons wh ...
... and how much energy does it have? To find frequency, use first formula, c=lambda x frequency Frequency = 3x 108 m/s / 700 x 10-9 meters = 4.29 x 1014 Hz To find Energy, plug it into the second formula: E = hf = (6.6 x 10-34) x (4.29 x 1014 Hz) = 2.82 x 10-19 Joules What is Fire? Excited electrons wh ...
Chap 2 Solns
... 2.4 (a) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (b) Two important refinements resulting from the wave-mechanical atomic model are (1) that ...
... 2.4 (a) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (b) Two important refinements resulting from the wave-mechanical atomic model are (1) that ...
Electron configuration Jeopardy
... 400 – Why don’t you see a baseball travel in waves? Wave nature is inversely related to mass. So if something is big, you don’t see the wave characteristic. 500 – What is the difference between a continuous spectrum and a line spectrum and give an example of where you could find each. Continuous spe ...
... 400 – Why don’t you see a baseball travel in waves? Wave nature is inversely related to mass. So if something is big, you don’t see the wave characteristic. 500 – What is the difference between a continuous spectrum and a line spectrum and give an example of where you could find each. Continuous spe ...
Prentice Hall Chemistry Worksheets
... 5. the amount of energy required to move an electron from its present energy level to the next higher one ...
... 5. the amount of energy required to move an electron from its present energy level to the next higher one ...
5 ELECTRONS IN ATOMS Vocabulary Review Name ___________________________
... 5. the amount of energy required to move an electron from its present energy level to the next higher one ...
... 5. the amount of energy required to move an electron from its present energy level to the next higher one ...
Ch.5 VocabReview
... 5. the amount of energy required to move an electron from its present energy level to the next higher one ...
... 5. the amount of energy required to move an electron from its present energy level to the next higher one ...
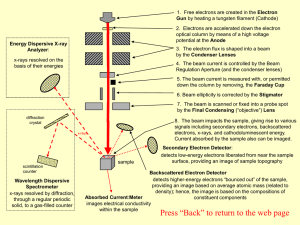
Primary electrons make random elastic and inelastic collision either
... will suffer a “quantum jump” to a low energy state, which will make emission of X-ray photon, and it would be all possible energy up to E0… Secondary electron, (<50 eV, normally around 2-6 eV, larger than sample’s work function) excitations result to loose bound valence electrons, which are promoted ...
... will suffer a “quantum jump” to a low energy state, which will make emission of X-ray photon, and it would be all possible energy up to E0… Secondary electron, (<50 eV, normally around 2-6 eV, larger than sample’s work function) excitations result to loose bound valence electrons, which are promoted ...
Unit 2 Intro Worksheet - Coral Gables Senior High
... 2. Why are you unable to observe the wavelike motion of a soccer ball as it is kicked toward a goal? 3. What is the quantum mechanical model? 4. Explain what is meant by the Heisenberg uncertainty principle. 5. Explain the three principles that govern the electron configuration in an atom. Matching ...
... 2. Why are you unable to observe the wavelike motion of a soccer ball as it is kicked toward a goal? 3. What is the quantum mechanical model? 4. Explain what is meant by the Heisenberg uncertainty principle. 5. Explain the three principles that govern the electron configuration in an atom. Matching ...
L-J Chemistry 1 Quiz 25 1 A property that depends on the amount of
... A cube that is 10 dm on each side =(equals 1 liter) Atomic mass of a substance in grams Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom when removing an electron Group of atoms held together by covalent bon ...
... A cube that is 10 dm on each side =(equals 1 liter) Atomic mass of a substance in grams Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom when removing an electron Group of atoms held together by covalent bon ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.