e - Purdue Physics - Purdue University
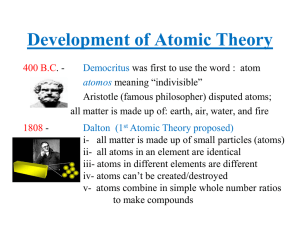
... The results of many experiments collectively suggest that all matter is made up of small, indivisible units which have a unique identity. Study of chemistry suggests a number of elementary substances (elements) that show unique chemical behavior. These elements are made up of identical tiny particle ...
... The results of many experiments collectively suggest that all matter is made up of small, indivisible units which have a unique identity. Study of chemistry suggests a number of elementary substances (elements) that show unique chemical behavior. These elements are made up of identical tiny particle ...
File - Science With BLT
... ____ 27. Which of the following is NOT an example of a molecular formula? a. H2O c. NH3 b. B d. O2 ____ 28. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle ...
... ____ 27. Which of the following is NOT an example of a molecular formula? a. H2O c. NH3 b. B d. O2 ____ 28. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Avogadro principle ...
First Semester Honors Chemistry Exam Review (2011
... 51. What is a positive ion? A negative ion? 52. What are Valence electrons? 53. How many valence electrons are in Group 1, Group 2, etc… 54. The electrostatic attraction between positively charged nuclei and negatively charged electrons permits two atoms to be held together by a(n)… 55. What is the ...
... 51. What is a positive ion? A negative ion? 52. What are Valence electrons? 53. How many valence electrons are in Group 1, Group 2, etc… 54. The electrostatic attraction between positively charged nuclei and negatively charged electrons permits two atoms to be held together by a(n)… 55. What is the ...
Atomic Structure and Quantum Theory
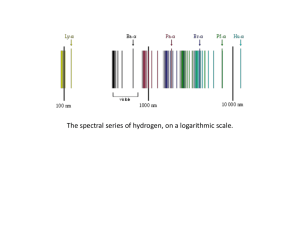
... Planck and Blackbody Radiation Einstein and the Photoelectric Effect Spectra Quantization ...
... Planck and Blackbody Radiation Einstein and the Photoelectric Effect Spectra Quantization ...
Problem Set 05
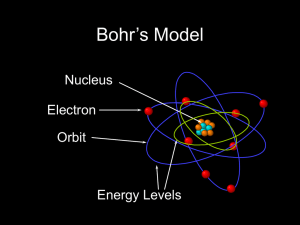
... A2. Spiral death time of an atom (in five easy steps): In Rutherford's planetary model of the atom electrons orbit around a very small massive nucleus. Classically, such an atom will have a finite lifetime due to radiative energy loss of the electrons, causing them to spiral in towards the nucleus. ...
... A2. Spiral death time of an atom (in five easy steps): In Rutherford's planetary model of the atom electrons orbit around a very small massive nucleus. Classically, such an atom will have a finite lifetime due to radiative energy loss of the electrons, causing them to spiral in towards the nucleus. ...
Midterm Review Sample Content Questions
... 20. Bohr is known for the “planetary model” of the atom – what does this mean? 21. What is the most accepted theory of the atom used in chemistry today? ...
... 20. Bohr is known for the “planetary model” of the atom – what does this mean? 21. What is the most accepted theory of the atom used in chemistry today? ...
CHEMISTRY 1A
... a. The quantum number, n, describes the _________and _________of an atomic orbital. b. The shape of an atomic orbital is given by the quantum number _________. c. The maximum number of orbitals that may be associated with the following set of quantum numbers n = 5 and l = 3 is _________. d. The maxi ...
... a. The quantum number, n, describes the _________and _________of an atomic orbital. b. The shape of an atomic orbital is given by the quantum number _________. c. The maximum number of orbitals that may be associated with the following set of quantum numbers n = 5 and l = 3 is _________. d. The maxi ...
27-3 A Photoelectric Effect Example
... (b) As we discussed in Exploration 27.2, the maximum kinetic energy of the emitted electrons is related to the minimum voltage across the two plates needed to stop the electrons from reaching the second plate (this is known as the stopping potential). In this case, the stopping potential is 1.40 V, ...
... (b) As we discussed in Exploration 27.2, the maximum kinetic energy of the emitted electrons is related to the minimum voltage across the two plates needed to stop the electrons from reaching the second plate (this is known as the stopping potential). In this case, the stopping potential is 1.40 V, ...
ap chemistry review – multiple choice
... Questions 15-18 refer to the following (a) Heisenbery uncertainty principle (b) Pauli exclusion principle (c) Hund’s rule (principle of maximum multiplicity) (d) Shielding effect (e) Wave nature of matter 15. Can be used to predict that a gaseous carbon atom in it ground state is paramagnetic 16. E ...
... Questions 15-18 refer to the following (a) Heisenbery uncertainty principle (b) Pauli exclusion principle (c) Hund’s rule (principle of maximum multiplicity) (d) Shielding effect (e) Wave nature of matter 15. Can be used to predict that a gaseous carbon atom in it ground state is paramagnetic 16. E ...
CH1710 HW#7 (2017)-Quanta, electron config
... 3. Cobalt-60 is a radioactive isotope used to treat cancers of the brain and other tissues. A gamma ray emitted by an atom of the isotope has an energy of 1.33 MeV (million electron volts). a. If 1 eV= 1.602 x 10-19 J, what is the frequency (in Hz) of this gamma ray ? ...
... 3. Cobalt-60 is a radioactive isotope used to treat cancers of the brain and other tissues. A gamma ray emitted by an atom of the isotope has an energy of 1.33 MeV (million electron volts). a. If 1 eV= 1.602 x 10-19 J, what is the frequency (in Hz) of this gamma ray ? ...
The Quantum Atom (section 18)
... So we can see that the kinetic energy is half the size of the potential energy. K.E. is positive while P.E. is negative. Total energy = K.E. + P.E = -e2/8πε0r ...
... So we can see that the kinetic energy is half the size of the potential energy. K.E. is positive while P.E. is negative. Total energy = K.E. + P.E = -e2/8πε0r ...
Quantum Notes
... • Matter can gain or lose energy only in small, specific amounts called quanta •A quantum is the minimum amount of energy that can be gained or lost by an atom ...
... • Matter can gain or lose energy only in small, specific amounts called quanta •A quantum is the minimum amount of energy that can be gained or lost by an atom ...
Rutherford–Bohr model
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.