* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download e - Purdue Physics - Purdue University
Density functional theory wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Bremsstrahlung wikipedia , lookup
James Franck wikipedia , lookup
Wave–particle duality wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Tight binding wikipedia , lookup
Electron configuration wikipedia , lookup
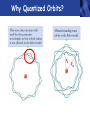
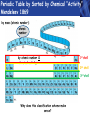
PHYS219 Fall semester 2014 Lecture 26: Electron Energy Levels in the Hydrogen Atom Dimitrios Giannios Purdue University Gas Discharge Tube – gases produce Discrete Wavelengths (late 1800s) Glass tube, evacuated and back-filled with gas Glass prism light is dispersed Focus on light emitted from Hydrogen discharge tube Balmer’s empirical formula (1884) explains the observed visible wavelengths from hydrogen gas with high accuracy n=7 n=6 n=5 n=4 é1 1ù = R H ê - ú ; n = 3, 4,5... êë22 n 2 úû l 1 RH = 1.097 × 107 m-1 n=3 The Bohr Model for the electronic energies in atomic Hydrogen To better understand the origin of Balmer’s empirical formula, Bohr formulated a theory of the hydrogen atom in 1911. http://www.anonymousartofrevolution.com/2012/08/great-people.html The Hydrogen Atom: 1e- and 1p Attractive Force via Coulomb’s Law ? ? ? e- p e- Bohr’s prediction: ao ~ 0.0529 nm (52.9 pm) (5.29×10-11 m) Electrically Neutral H Atom p r=ao symbol charge mass neutron n 0 (zero) proton P +1.6 x 10-19 C electron e-1.6 x 10-19 C 1.675 x 10-27 kg 1.673 x 10-27 kg 9.11 x 10-31 kg Note: 1 C = 1 Coulomb Bohr Model (1911): Assumptions (hybrid model combining classical and quantum physics) • Electron moves in special circular orbits – stationary states • Only certain orbits are allowed; quantization of angular momentum determines radius of orbit: r=n2ao; n=positive non-zero integer • Electron gives off no radiation when in stationary orbit • Radiation only emitted when electron makes transition from one stationary orbit to another allowed electron orbits Nucleus (1 proton for H) Bohr’s model for light emission from H Quantized electron orbits “ground state” n=1 rn = n2 a0 ao = 0.0529nm rn n=2 n=3 ....... nucleus Using this model, you can derive Balmer’s empirical formula Why Quantized Orbits? rn rm Each orbit gives rise to a discrete energy level Energy Newtonian Physics n=∞ n=3 n=2 n=1 E=0 Quantum Physics ground state same result that Einstein proposed ΔE = Ef - Ei emitted photon bound states Bohr predicts the allowed electron energy levels for H atom Prediction of Bohr’s Model: Energy E=0 n=3 n=2 n=1 E3=-1.51 eV E2=-3.40 eV E1=-13.6 eV ground state Example – Balmer Lines Transition from n=7 n=6 n=5 n=4 ΔE = En - Em where E n = DE = E0 - E0 E0 n 2 and E m = - = hf = h E0 m c m2 n 2 l é 1 1 E0é 1 1ù 1ù ê = - ú = RH ê - ú 2 2 2 êë m l hc êë m n úû n 2 úû 2 ; E 0 = 13.6eV n=3 to the n=2 level Example •An electron in the n = 3 orbit has an energy of -13.6 eV/32= -1.51 eV •An electron in the n = 2 orbit has an energy of -13.6 eV/22= -3.40 eV •An electron that drops from n=3 to n=2 gives up 3.40 eV - 1.51 eV=1.89 eV of energy, the energy of the red-color photon. •The 656 nm emission line from H has a frequency f=4.57×1014 Hz. A photon of this color has an energy of hf= 3.03×10−19 J (1.89 eV). Atomic Structure The results of many experiments collectively suggest that all matter is made up of small, indivisible units which have a unique identity. Study of chemistry suggests a number of elementary substances (elements) that show unique chemical behavior. These elements are made up of identical tiny particles called atoms (Greek for “without division”). Classify the Elements by Mass - Dalton’s contribution (1804) light • Hydrogen (H) • Helium (He) • Lithium (Li) • Beryllium (Be) • Boron (B) • Calcium (Ca) heavy • etc. Periodic Table by Sorted by Chemical “Activity” Mendeleev 1869 by mass (atomic number): atomic number by atomic number & chemical activity: 1st shell 2nd shell 3rd shell Why does this classification scheme make sense? Each chemical element has fixed number of electrons and protons Example: C has 6 protons, 6 electrons. Electronic energy states in isolated atom are QUANTIZED E=0 p s p Allowed Energies s Chemistry of each element determined by most weakly bound electron energy states Check out http://www.colorado.edu/physics/2000/applets/a2.html Electronic Energy Shells 1st 20 Chemical Elements Bohr’s Model (1911) 1st shell holds 2 e’s Source: www.chemicool.com/elements/ 2nd shell holds 8 e’s Schrödinger’s Model (1926) 3rd shell holds 8 e’s Z= Number of protons http://www.jcrystal.com/steffenweber/ gallery/sh/orbitals4.htm