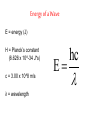* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Arrangement of Electrons In Atoms
Quantum machine learning wikipedia , lookup
Coherent states wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Quantum group wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Quantum key distribution wikipedia , lookup
Chemical bond wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Quantum teleportation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
EPR paradox wikipedia , lookup
History of quantum field theory wikipedia , lookup
Canonical quantization wikipedia , lookup
Quantum state wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Hidden variable theory wikipedia , lookup
Electron scattering wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Particle in a box wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Molecular orbital wikipedia , lookup
Tight binding wikipedia , lookup
Matter wave wikipedia , lookup
Hydrogen atom wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Wave–particle duality wikipedia , lookup
Atomic theory wikipedia , lookup
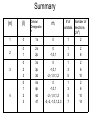
Arrangement of Electrons In Atoms Objectives 1. Explain relationship between speed, wavelength, frequency of electromagnetic radiation 2. Discuss the wave-particle duality of light 3. How the photoelectric effect and line-emission spectrum of hydrogen helped develop the atomic model 4. Describe the Bohr model of the atom Seeing the Light c = 3.00 x 10^8 m/s (speed of light) Consists of particles called photons Also has wave-like properties Wavelength (λ) - distance between two corresponding points on adjacent waves Frequency (v)- # of waves that pass a point in any given amount of time The Visible Spectrum • Between 380nm – 750nm • Shorter wavelength is more powerful (ionizing) • Blue has shortest wavelength Energy of a Wave E = energy (J) H = Planck’s constant (6.626 x 10^-34 J*s) c = 3.00 x 10^8 m/s λ = wavelength E hc The Eelectromagnetic Spectrum Bohr Model of the Atom • Electrons orbit nucleus at fixed distances • Electrons can only have fixed energies at these distances • Each orbit called a shell and is assigned a quantum number Shells • Electrons in shell n = 1 have lowest energy • Each shell can hold up to 2n2 electrons • Innermost shell is filled first Quantum Model of the Atom Objectives 1. 2. 3. Explain how Heisenberg uncertainty principle and Shroedinger wave equation led to idea of atomic orbitals List 4 quantum numbers & their significance Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per energy sublevel, and the number of orbitals per main energy level Heisenberg Uncertainty Principle It is impossible to simultaneously determine the location and velocity of an electron Schrödinger Wave Equation Quantum Theory – describes mathematically the wave properties of electrons and other very small particles Principle Quantum Numbers • Symbolized by n • Value of n are positive integers (1,2,3 etc) • As n increases, so does its energy and distance from nucleus • More than one e- can have the same n value • Also called shells or main energy level • Total number of orbitals in a shell = n2 Angular Momentum Quantum Number • • • • • Symbolized by l Indicate the shape of the orbital Also called sublevels Number of shapes equal to n Value of l are zero and all positive integers less than or equal to (n - 1) Example: n = 2; l = 0 or l = 1 • Each integer is assigned a letter Example: 0 = s; 1 = p; 2 = d; 3 = f • n = 2; there are two sublevels s and p • Each orbital is designated by its principle quantum number and letter of the sublevel Example: 1s, 2s or 2p Magnetic Quantum Number • Symbolized by m • Represents orientation of orbital around the nucleus • s orbital is spherical; has one orientation • p orbital is dumbbell shaped; 3 orientations px, py, pz • m = -1, m = 0, or m = 1 Summary (n) (l) Orbital Designatio n ml 1 0 1s 0 1 2 2 0 1 2s 2p 0 -1,0,1 1 3 2 6 3 0 2 3 3s 3p 3d 0 -1,0,1 -2,-1,0,1,2 1 3 5 2 6 10 0 1 2 3 4s 4p 4d 4f 0 -1,0,1 -2,-1,0,1,2 -3,-2,-1,0,1,2,3 1 3 5 7 2 6 10 14 4 # of Number of orbitals electrons (2n2) Spin Quantum Number • Only two possible values • -1/2 or +1/2 • Any single orbital can hold only two electrons