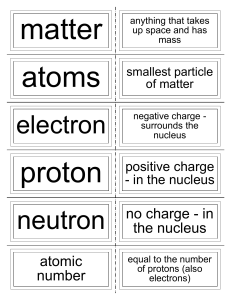
Unit 6: Covalent Bonds Review KEY
... Label the following bonds as being nonpolar covalent, polar covalent or ionic, based on electronegativity difference: ...
... Label the following bonds as being nonpolar covalent, polar covalent or ionic, based on electronegativity difference: ...
Test 4
... 12. Identify polar or nonpolar covalent bonds. 13. Identify largest or smallest electronegativity of elements. 14. Write electron configuration of atoms and ions. 15. Lewis Structure is also called Electron Dot Structure. Be able to draw Electron Dot Structure for any atom or molecule. 16. Identify ...
... 12. Identify polar or nonpolar covalent bonds. 13. Identify largest or smallest electronegativity of elements. 14. Write electron configuration of atoms and ions. 15. Lewis Structure is also called Electron Dot Structure. Be able to draw Electron Dot Structure for any atom or molecule. 16. Identify ...
Lecture 2 - The Chemistry of Life
... • In a nonpolar covalent bond, the atoms share the electron equally • In a polar covalent bond, one atom is more electronegative, and the atoms do not share the electron equally • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule ...
... • In a nonpolar covalent bond, the atoms share the electron equally • In a polar covalent bond, one atom is more electronegative, and the atoms do not share the electron equally • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule ...
models of molecular compounds
... MODELS OF MOLECULAR COMPOUNDS Introduction: Molecular shape determines a compound’s boiling point, freezing/melting point, viscosity and the nature of its reactions. The geometry of a small molecule can be predicted by examining the central atom and identifying the number of atoms bonding to it and ...
... MODELS OF MOLECULAR COMPOUNDS Introduction: Molecular shape determines a compound’s boiling point, freezing/melting point, viscosity and the nature of its reactions. The geometry of a small molecule can be predicted by examining the central atom and identifying the number of atoms bonding to it and ...
Chemical Compounds
... S Take your ion and find someone you can bond with S Attempt to create the compound H2O, MgCl2..and so on S We will come together as a class and try to figure out if you ...
... S Take your ion and find someone you can bond with S Attempt to create the compound H2O, MgCl2..and so on S We will come together as a class and try to figure out if you ...
Molecular Geometry
... • Use the Lewis Structure to determine the geometry of the molecule. • Focus only on the CENTRAL atom for all molecules! Count the number of electron regions. • Look for REGIONS WHERE ELECTRONS ARE FOUND rather than number of bonds. (Ex: Double and Triple bonds count as 1 region) ...
... • Use the Lewis Structure to determine the geometry of the molecule. • Focus only on the CENTRAL atom for all molecules! Count the number of electron regions. • Look for REGIONS WHERE ELECTRONS ARE FOUND rather than number of bonds. (Ex: Double and Triple bonds count as 1 region) ...
12-3: Lewis Structures
... around the chemical symbol All atoms want to achieve a noble gas configuration o Octet Rule—most elements will be surrounded by 8 dots, representing noble gas configuration Hydrogen is full with 2 electrons (2 dots on one side)—so it is like helium Draw the Lewis structures for: H Ca N F ...
... around the chemical symbol All atoms want to achieve a noble gas configuration o Octet Rule—most elements will be surrounded by 8 dots, representing noble gas configuration Hydrogen is full with 2 electrons (2 dots on one side)—so it is like helium Draw the Lewis structures for: H Ca N F ...
Lab- Molecular Geometry PSI Chemistry
... 1. Write the Lewis electron-dot symbol for each of the following atoms: hydrogen, boron, nitrogen, silicon, sulfur and bromine. 2. What information about a molecule does the Lewis structure provide? What information is neither shown nor implied in the Lewis structure? 3. There are several exceptions ...
... 1. Write the Lewis electron-dot symbol for each of the following atoms: hydrogen, boron, nitrogen, silicon, sulfur and bromine. 2. What information about a molecule does the Lewis structure provide? What information is neither shown nor implied in the Lewis structure? 3. There are several exceptions ...
CHEM 1411 CHAPTER 9
... bonds. There are molecules containing polar bonds but the molecule as a whole is non-polar. The overall polarity depends on the geometry of the molecule. The following rules are to be kept in mind to decide a molecule polar or nonpolar Symmetric molecules containing same kind of atoms around the c ...
... bonds. There are molecules containing polar bonds but the molecule as a whole is non-polar. The overall polarity depends on the geometry of the molecule. The following rules are to be kept in mind to decide a molecule polar or nonpolar Symmetric molecules containing same kind of atoms around the c ...
chemical bonding
... • Chemical compounds are formed by the bonding of two or more atoms. A stable bonding forms when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms called a chemical bond. Chemical bonds are classified in t ...
... • Chemical compounds are formed by the bonding of two or more atoms. A stable bonding forms when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms called a chemical bond. Chemical bonds are classified in t ...
Unit 9
... The arrangement of electron domains about the central atom or ion is the electron domain geometry The molecular geometry is the arrangement of only the atoms in a molecule or ion (nonbonding pairs are not included) Nonbonding pairs are larger than bonding pairs and have greater repulsions (decrease ...
... The arrangement of electron domains about the central atom or ion is the electron domain geometry The molecular geometry is the arrangement of only the atoms in a molecule or ion (nonbonding pairs are not included) Nonbonding pairs are larger than bonding pairs and have greater repulsions (decrease ...
Chapter 6 Class Notes CHEM
... 2. What is the relationship between bond energy and bond length? 3. What type of bond would you expect to exist between an atom of Calcium, Ca and an atom of Oxygen, O? 4. What type of bond would you expect to exist between two atoms of Hydrogen and an atom of Oxygen? 5. Which type of substance, ion ...
... 2. What is the relationship between bond energy and bond length? 3. What type of bond would you expect to exist between an atom of Calcium, Ca and an atom of Oxygen, O? 4. What type of bond would you expect to exist between two atoms of Hydrogen and an atom of Oxygen? 5. Which type of substance, ion ...
Experiment 30A3- Structures
... number of total electrons and will therefore have one element with an incomplete octet. c. Elements in period 3 and below are able to expand their octet and accommodate more than eight electrons in their valence shells due to the availability of vacant d-orbitals. ...
... number of total electrons and will therefore have one element with an incomplete octet. c. Elements in period 3 and below are able to expand their octet and accommodate more than eight electrons in their valence shells due to the availability of vacant d-orbitals. ...
CHEM 1411 CHAPTER 9
... It is not always necessary that a molecule must be polar if it contains polar bonds. There are molecules containing polar bonds but the molecule as a whole is non-polar. The overall polarity depends on the geometry of the molecule. The following rules are to be kept in mind to decide a molecule pola ...
... It is not always necessary that a molecule must be polar if it contains polar bonds. There are molecules containing polar bonds but the molecule as a whole is non-polar. The overall polarity depends on the geometry of the molecule. The following rules are to be kept in mind to decide a molecule pola ...
Chapter 4 REVIEW
... Chapter 4 REVIEW 1. Draw Lewis symbols for atoms of the following elements and predict their bonding capacity: (a) calcium (d) silicon (b) chlorine (e) sulfur (c) phosphorus ...
... Chapter 4 REVIEW 1. Draw Lewis symbols for atoms of the following elements and predict their bonding capacity: (a) calcium (d) silicon (b) chlorine (e) sulfur (c) phosphorus ...
Homework Geochem Test Review
... 12. What part of an atom is negative? _________ What part is positive? _________ What part is neutral? ___________ 13. What is the atomic mass? Why don’t we count the electrons when determining the atomic mass? ...
... 12. What part of an atom is negative? _________ What part is positive? _________ What part is neutral? ___________ 13. What is the atomic mass? Why don’t we count the electrons when determining the atomic mass? ...
CHEM 1515 1 Spring 2001 Chem 1515 Problem Set #1 Spring 2001
... a) The H–N–H bond angle is 107.5 ˚ in NH 3. Ammonia has three bonding pairs of electrons and one nonbonding pair of electrons. The electron-pair geometry about a central atom with four bonding pairs of electrons and no lone pairs is tetrahedral with bond angles of 109.5˚. The replacement of one bond ...
... a) The H–N–H bond angle is 107.5 ˚ in NH 3. Ammonia has three bonding pairs of electrons and one nonbonding pair of electrons. The electron-pair geometry about a central atom with four bonding pairs of electrons and no lone pairs is tetrahedral with bond angles of 109.5˚. The replacement of one bond ...
Masterton and Hurley - Chapter 7
... 1. Definitions chemical bond – an attraction strong enough to hold 2 atoms or ions together ...
... 1. Definitions chemical bond – an attraction strong enough to hold 2 atoms or ions together ...