* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 2 - The Chemistry of Life
Survey
Document related concepts
Transcript
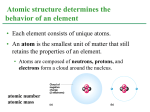
Lecture 2: The Chemistry of Life In this lecture: • Matter, atoms, and the periodic table • Chemical bonding – Ionic vs. covalent bonds – Hydrogen bonds and Van der Waals forces • Polarity • Electronegativity What is “stuff” made of? • Matter – Anything that takes up space and has mass (or weight) – Makes up everything we see, touch, feel, and hear in the universe – (Dark matter is another thing entirely) What is “matter” made of? Matter is made up of elements. An element is “a substance that cannot be broken down to other substances by chemical reactions” Potassium, hydrogen, carbon, phosphorus Compounds are substances made of two or more elements The properties of a compound are not related to the properties of its elements The periodic table is very carefully arranged. We shall see why soon… Matter = atoms = elements Compounds = 2+ elements The elements of life • About 20-25% of the elements are necessary to life • Carbon, hydrogen, nitrogen, and oxygen make up 96% of living beings • Phosphorus, calcium, potassium, and sulfur make up most of the other 4% • There are some trace elements that are necessary for life, but needed in very minute amounts – Zinc in DNA regulation, silicon in shell formation, iron in blood What is matter made of? • Matter is made of atoms • An atom is the smallest unit of matter that still retains the properties of an element – Atom means “indivisible” in Greek What are atoms made of? • Three subatomic particles go into an atom – Proton (positive charge) – Neutron (neutral/no charge) – Electron (negative charge) • Neutrons and protons form the atomic nucleus • Electrons form a cloud around the nucleus • Neutron mass and proton mass are almost identical and are measured in daltons A helium atom Is this what an atom really looks like? Atomic Number and Atomic Mass • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • Atomic mass, the atom’s total mass is the sum of protons plus neutrons in the nucleus Electron positions • Electrons are arranged in “shells” around the nucleus • 2 electrons in the innermost shell, 8 in all the subsequent shells • The number of electrons an atom has is equal to the number of protons – (7 positive protons + 7 negative electrons = neutral charge) – Neutrons just hang out Figure 2.9 Hydrogen 1H Mass number First shell 2 He 4.00 Atomic number Helium 2He Element symbol Electron distribution diagram Lithium 3Li Beryllium 4Be Boron 5B Carbon 6C Nitrogen 7N Oxygen 8O Fluorine 9F Neon 10Ne Silicon 14Si Phosphorus 15P Sulfur 16S Chlorine 17Cl Argon 18Ar Second shell Sodium 11Na Third shell Magnesium Aluminum 12Mg 13Al Valence electrons • Valence electrons are those in the outermost shell • The chemical behavior of an atom is mostly determined by the valence electrons • Elements with a full valence shell are chemically inert Diagram of valence electrons Let’s draw a sulfur atom • • • • • What is the atomic number of sulfur? How many protons? How many neutrons? How many electrons? Where do the electrons go? Solution: A sulfur atom Isotopes • All atoms of an element have the same number of protons but may differ in number of neutrons • Isotopes are two atoms of an element that differ in number of neutrons • Radioactive isotopes decay spontaneously, giving off particles and energy Radioactive decay of isotopes • Half-life is the time it takes half of the isotopes in a compound to decay into normal atoms • The ratio of carbon-14 to carbon-12 can be used to date biological samples • Some applications of radioactive isotopes in biological research are – Dating fossils – Tracing atoms through metabolic processes – Diagnosing medical disorders Summary of an atom Chemical Bonds • Atoms with incomplete valence shells (<8) “want” to have filled shells • Atoms will bond with each other to fill their shells, forming a chemical bond Figure 2.11-3 Hydrogen atoms (2 H) Hydrogen has one proton and one valence electron It wants two to completely fill its inner shell, and so will bond Hydrogen molecule (H2) Bonding • A molecule consists of two or more atoms held together by covalent bonds • Four types of bonds: covalent, ionic, metallic, hydrogen (sometimes) • Covalent and hydrogen most common in biology Ionic Bonding • Ionic bonding – electron is donated Ionic Bonding • Atoms sometimes strip electrons from their bonding partners • An example is the transfer of an electron from sodium to chlorine • After the transfer of an electron, both atoms have charges • A charged atom (or molecule) is called an ion • A cation is a positively charged ion • An anion is a negatively charged ion • An ionic bond is an attraction between an anion and a cation Covalent Bonding • Covalent bonding – electron is shared • Some examples of covalent bonds Bonding in covalent bonds • A single bond is the sharing of one pair of valence electrons • A double bond is the sharing of two pairs of valence electrons Polarity in covalent bonds • In a nonpolar covalent bond, the atoms share the electron equally • In a polar covalent bond, one atom is more electronegative, and the atoms do not share the electron equally • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule Electronegativity • Atoms in a molecule attract electrons to varying degrees • Electronegativity is an atom’s attraction for the electrons • The more electronegative an atom, the more strongly it pulls shared electrons toward itself Electronegativity Electronegativity increases in this direction Weak Chemical Bonds • Most of the strongest bonds in organisms are covalent bonds that form a cell’s molecules • Weak chemical bonds, such as ionic bonds and hydrogen bonds, are also important • Weak chemical bonds reinforce shapes of large molecules and help molecules adhere to each other Weak Chemical Bonds – Hydrogen Bonds • A hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom • In living cells, the electronegative partners are usually oxygen or nitrogen atoms Weak Chemical Bonds: Van der Waals force • Polar molecules have “hot spots” of negative or positive charge where electrons are unevenly distributed • Van der Waals interactions are attractions between molecules that are close together as a result of these charges “This force comes from fluctuations in charge distributions between neighboring molecules, which need not be polar; their charge fluctuations naturally fall into synch, creating an attractive force.” How Geckos stick on der Waals • Geckos use Van der Waals interactions between their toe hairs and a wall to ‘stick’ Chemical Reactions: making and breaking bonds • The starting molecules of a chemical reaction are called reactants • The final molecules of a chemical reaction are called products Photosynthesis, a chemical reaction • Plants use sunlight to create an essential building block • Sunlight powers the conversion of carbon dioxide and water to glucose and oxygen 6 CO2 + 6 H20 → C6H12O6 + 6 O2 What do my multivitamins do in the body? • The FDA officially recognizes 12 ‘essential’ minerals: calcium, magnesium, zinc, selenium, copper, manganese, chromium, molybdenum, and chloride • Silicon, boron, nickel, vanadium, and lead may play a biological role, but aren’t ‘essential’ – The role of these hasn’t been well defined – For a lot of these, we don’t know exactly what they do, so the FDA is hands-off Biological organisms and elements Mono Lake, CA: The ‘Aliens’ of Mono Lake • Mono Lake has no natural outlets – Chemicals enter, but don’t leave – HUGE buildup of toxic waste chemicals and salt – No fish, only brine shrimp can live there • In December 2010 NASA announced the discovery of a bacteria that can use arsenic – Arsenic is normally VERY toxic to living things!! – It replaces phosphorus in biochemical reactions, but is far less stable – Anything that accidentally includes arsenic (DNA, ATP, etc.) tends to break down very fast • These bacteria can stably integrate arsenic in place of phosphorus into their DNA • Use large storage vacuoles to isolate arseniccontaining molecules away from important cellular machinery Controversey • 2012 – scientists begin refuting NASA’s findings • NASA scientists fed the bacteria salts that contained trace amounts of phosphorus, which may have allowed the bacteria to live • No arsenic found in the DNA after all http://www.scientificamerican.com/article.cfm?id=study-fails-to-confirm-existence Vocabulary • • • • • • Matter Element Compound Atom Molecule Protons, neutrons, electrons • Nucleus • Atomic number • Isotope • Potential energy, kinetic energy • Valence electrons • Electron shells • Electronegativity • Covalent bond, ionic bond • Hydrogen bond, Van der Waals force • Products, reactants • Polarity