Midterm Review
... Describe the trend for electronegativity on the periodic table (which elements have high electronegativities and which have low electronegativitities). ...
... Describe the trend for electronegativity on the periodic table (which elements have high electronegativities and which have low electronegativitities). ...
Worksheet on Ionic and Atomic Size Trends
... 12. The sodium ion is smaller than the atom, because when sodium loses its valence electron to form an ion, it also loses its 3rd energy level. 13. The chlorine ion is larger than the chlorine atom, because adding an additional electron to the 3 rd energy level causes the energy level to expand beca ...
... 12. The sodium ion is smaller than the atom, because when sodium loses its valence electron to form an ion, it also loses its 3rd energy level. 13. The chlorine ion is larger than the chlorine atom, because adding an additional electron to the 3 rd energy level causes the energy level to expand beca ...
Chemical Properties of Water - Part 2
... Many substances, such as household sugar, dissolve in water. That is, their molecules separate from each other, each becoming surrounded by water molecules. ...
... Many substances, such as household sugar, dissolve in water. That is, their molecules separate from each other, each becoming surrounded by water molecules. ...
chapter2 2012 (no naming)
... • When an ionic compound dissolves in water, the ions are released from each other • conductivity – the ions in a solution support the transmission of an electric current • Strong electrolytes – solutions that are very good conductors • Weak electrolytes – solutions that are poor conductors • Nonele ...
... • When an ionic compound dissolves in water, the ions are released from each other • conductivity – the ions in a solution support the transmission of an electric current • Strong electrolytes – solutions that are very good conductors • Weak electrolytes – solutions that are poor conductors • Nonele ...
Pre-Knowledge: Chemistry and Physics Vocabulary Atomic Number
... A positively or negatively charged atom or molecule. There are two kinds of ions, anions and cations, which are named for the sign of the net charge. Anions have gained one or more electrons, giving them more negatively charged electrons than positively charged protons, for an overall negative charg ...
... A positively or negatively charged atom or molecule. There are two kinds of ions, anions and cations, which are named for the sign of the net charge. Anions have gained one or more electrons, giving them more negatively charged electrons than positively charged protons, for an overall negative charg ...
FE Review Chemistry - UTSA College of Engineering
... • Element: a substance only composed of one type of atom • Isotope: element with the same number of protons but different atomic masses ...
... • Element: a substance only composed of one type of atom • Isotope: element with the same number of protons but different atomic masses ...
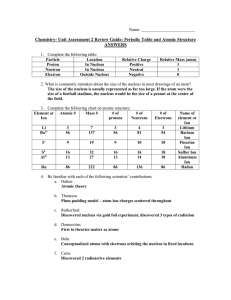
Chem Unit 2 Review Guide ANSWERS
... Atomic mass unit. Equal to the mass of a proton or neutron. 14.) What is a radioactive isotope? An unstable atom which decay (break down) and give off radioactive energy. 15.) What makes an atom unstable? An imbalance in the ratio of protons to neutrons. The farther this ratio gets from 1:1, the mor ...
... Atomic mass unit. Equal to the mass of a proton or neutron. 14.) What is a radioactive isotope? An unstable atom which decay (break down) and give off radioactive energy. 15.) What makes an atom unstable? An imbalance in the ratio of protons to neutrons. The farther this ratio gets from 1:1, the mor ...
Slide 1
... electrons are shared by two atoms. • Ionic bond: one or more electrons from one atom are removed and attached to another atom. • resulting in positive and negative ions which attract each other. ...
... electrons are shared by two atoms. • Ionic bond: one or more electrons from one atom are removed and attached to another atom. • resulting in positive and negative ions which attract each other. ...
Atom (A) or Ion
... 17. This substance is nitrogen. 18. This substance is carbon. 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny ind ...
... 17. This substance is nitrogen. 18. This substance is carbon. 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny ind ...
Atom (A) or Ion (I)
... 17. This substance is nitrogen. 18. This substance is carbon. 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny ind ...
... 17. This substance is nitrogen. 18. This substance is carbon. 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny ind ...
Atom (A) or Ion (I)
... 17. This substance is nitrogen. 18. This substance is carbon. 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny ind ...
... 17. This substance is nitrogen. 18. This substance is carbon. 19. This substance is an ion. 20. This substance is an isotope of carbon-12 (12C) 21. The Atomic Theory was first stated in the late 1700s. Which of the following is NOT part of this Atomic Theory? A) All elements are composed of tiny ind ...
Chemistry Review - Woodlawn School Wiki
... solution potassium sulfate and a precipitate fell out. Using balanced chemical equations, show work to find out what ion or ions were in my solution. 2) A 1.42-g sample of a pure compound, with formula M2SO4 , was dissolved in a water and treated with an excess of aqueous barium chloride, resulting ...
... solution potassium sulfate and a precipitate fell out. Using balanced chemical equations, show work to find out what ion or ions were in my solution. 2) A 1.42-g sample of a pure compound, with formula M2SO4 , was dissolved in a water and treated with an excess of aqueous barium chloride, resulting ...
Writing Formulas
... Binary compounds consist of two elements that may be ionic or covalent. The general rule is to put the least electronegative element first, the more electronegative element second and balance the charges to zero. ...
... Binary compounds consist of two elements that may be ionic or covalent. The general rule is to put the least electronegative element first, the more electronegative element second and balance the charges to zero. ...
File
... • Few compounds are able to conduct electricity in the solid state • BUT some conduct electricity when dissolved in water • These compounds are called electrolytes ...
... • Few compounds are able to conduct electricity in the solid state • BUT some conduct electricity when dissolved in water • These compounds are called electrolytes ...
Chemistry Exam Review
... sodium hydroxide reacts with hydrochloric acid to produce sodium chloride and water ...
... sodium hydroxide reacts with hydrochloric acid to produce sodium chloride and water ...
File
... • The result is that the positive metal ion is attracted not only to the negative non-metal ion it gave its electron(s) to, but also all the other non-metal ions. • This results in a crystal lattice structure. ...
... • The result is that the positive metal ion is attracted not only to the negative non-metal ion it gave its electron(s) to, but also all the other non-metal ions. • This results in a crystal lattice structure. ...
CHEMISTRY FINAL EXAM REVIEW SHEET
... Hydrogen is usually +1. Oxygen is usually –2. In a compound, the more electronegative element is given an oxidation number equal to its usual ionic charge. The sum of the oxidation numbers must equal the overall charge on the compound or ion. ...
... Hydrogen is usually +1. Oxygen is usually –2. In a compound, the more electronegative element is given an oxidation number equal to its usual ionic charge. The sum of the oxidation numbers must equal the overall charge on the compound or ion. ...
Draw atomic models showing the appropriate number of electrons
... 3. The electrical force of attraction that holds ions of opposite charge together 4. A chemical bond in which atoms are held together by their mutual attraction for two electrons they share 5. Type of bond that forms between two atoms of similar electronegativity when electrons are equally shared 6. ...
... 3. The electrical force of attraction that holds ions of opposite charge together 4. A chemical bond in which atoms are held together by their mutual attraction for two electrons they share 5. Type of bond that forms between two atoms of similar electronegativity when electrons are equally shared 6. ...
Define:
... 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi .01 47. Make the following conversions: a. 8961 m to ...
... 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi .01 47. Make the following conversions: a. 8961 m to ...
Atoms and the Periodic Table
... A negatively charged atom is called an Anion – it has more electrons than protons. ...
... A negatively charged atom is called an Anion – it has more electrons than protons. ...
First Semester Honors Chemistry Exam Review (2011
... 38. A spherical electron cloud surrounding an atomic nucleus would best represent which orbital (s)? 39. How many orbital shapes are in the first energy level? Second? Third? Fourth? What are they? 40. Both copper (atomic number 29) and chromium (atomic number 24) appear to break the pattern in the ...
... 38. A spherical electron cloud surrounding an atomic nucleus would best represent which orbital (s)? 39. How many orbital shapes are in the first energy level? Second? Third? Fourth? What are they? 40. Both copper (atomic number 29) and chromium (atomic number 24) appear to break the pattern in the ...
NOTES: 2.1 - Intro to Chemistry
... most compounds! ● EXAMPLE: 1 molecule of water, H2O, is the smallest unit of water possible; it consists of 2 hydrogen atoms & 1 oxygen atom bonded together. ...
... most compounds! ● EXAMPLE: 1 molecule of water, H2O, is the smallest unit of water possible; it consists of 2 hydrogen atoms & 1 oxygen atom bonded together. ...
Matter Unit - OG
... 1.) Are made up of only one type of atom. 2) Cannot be broken down into any simpler substances by normal physical or chemical means. 3) Periodic Table of Elements *Familiarize yourself w/ it *Know what those numbers mean! ...
... 1.) Are made up of only one type of atom. 2) Cannot be broken down into any simpler substances by normal physical or chemical means. 3) Periodic Table of Elements *Familiarize yourself w/ it *Know what those numbers mean! ...