The Atomic Zoo
... of a parallel-plate capacitor. By measuring the rate at which they drifted down and then the electric field strength required to make them hover, he was able to calculate the electrical charge on any droplet. He found that the charge was always a whole number multiple of a particular basic charge, “ ...
... of a parallel-plate capacitor. By measuring the rate at which they drifted down and then the electric field strength required to make them hover, he was able to calculate the electrical charge on any droplet. He found that the charge was always a whole number multiple of a particular basic charge, “ ...
Midterm Review.ppt - Chemistry R: 4(AE)
... • When electrons in an atom in an excited state fall to lower energy levels, energy is 1. absorbed, only 2. released, only 3. neither released nor absorbed 4. both released and absorbed ...
... • When electrons in an atom in an excited state fall to lower energy levels, energy is 1. absorbed, only 2. released, only 3. neither released nor absorbed 4. both released and absorbed ...
Unit 3 Test - hrsbstaff.ednet.ns.ca
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
Review 1st Qtr KEY
... 3. For an electron in an atom to change from the ground state to an excited state, a. energy must be released. b. energy must be absorbed. c. radiation must be emitted. d. the electron must make a transition from a higher to a lower energy level. ...
... 3. For an electron in an atom to change from the ground state to an excited state, a. energy must be released. b. energy must be absorbed. c. radiation must be emitted. d. the electron must make a transition from a higher to a lower energy level. ...
e - Purdue Physics - Purdue University
... •The 656 nm emission line from H has a frequency f=4.57×1014 Hz. A photon of this color has an energy of hf= 3.03×10−19 J (1.89 eV). ...
... •The 656 nm emission line from H has a frequency f=4.57×1014 Hz. A photon of this color has an energy of hf= 3.03×10−19 J (1.89 eV). ...
Periodic Table
... Ionized oil drops and watched them fall. First examined drop in falling at a terminal velocity without an electric field. He measured both the size of the particle and how fast it was falling. The size allowed him to quantify both the mass as well as the drag on the particle. Then he repeated the ex ...
... Ionized oil drops and watched them fall. First examined drop in falling at a terminal velocity without an electric field. He measured both the size of the particle and how fast it was falling. The size allowed him to quantify both the mass as well as the drag on the particle. Then he repeated the ex ...
Atoms
... Mention Begeman's role and history. The Rutherford Nuclear Atom Play: Mech. Univ. video “Atoms” Ch. 15. After the discovery and characterization of the electron, Thompson proposed a model of the atom in which electrons were dispersed like raisins in a uniform distribution of positive charge ...
... Mention Begeman's role and history. The Rutherford Nuclear Atom Play: Mech. Univ. video “Atoms” Ch. 15. After the discovery and characterization of the electron, Thompson proposed a model of the atom in which electrons were dispersed like raisins in a uniform distribution of positive charge ...
Transcript - the Cassiopeia Project
... Yet the electron couldn’t be in an orbit circling the nucleus either. Circular motion requires constant acceleration of the circling body to keep it from flying away. But the electron has charge and charged particles radiate light when they are accelerating. So an electron in a circular orbit would ...
... Yet the electron couldn’t be in an orbit circling the nucleus either. Circular motion requires constant acceleration of the circling body to keep it from flying away. But the electron has charge and charged particles radiate light when they are accelerating. So an electron in a circular orbit would ...
SOL PS3 Structure of the Atom by GA Tech
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
Thornton/Rex Chp 4 Structure of the Atom
... Ernest Rutherford, giving an uncharacteristic compliment to a theorist-Niels Bohr in this case. ...
... Ernest Rutherford, giving an uncharacteristic compliment to a theorist-Niels Bohr in this case. ...
CP-Chem Ch 3 PowerPoint(Atomic Theory
... atomic theory that he created using the laws of matter and previously known atomic theory • 1) All matter is composed of atoms • 2) All atoms of a given element are identical in size, mass, and other properties • 3) Atoms can not be divided, created or destroyed • 4) Atoms of different elements comb ...
... atomic theory that he created using the laws of matter and previously known atomic theory • 1) All matter is composed of atoms • 2) All atoms of a given element are identical in size, mass, and other properties • 3) Atoms can not be divided, created or destroyed • 4) Atoms of different elements comb ...
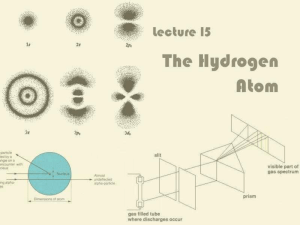
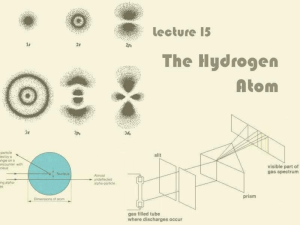
Lecture 15: The Hydrogen Atom
... One of the transitions in the Balmer series corresponds to the emission of red light ...
... One of the transitions in the Balmer series corresponds to the emission of red light ...
Electron
... • A subatomic particle of the nucleus of an atom without a charge that contributes to the mass of an atom. ...
... • A subatomic particle of the nucleus of an atom without a charge that contributes to the mass of an atom. ...
المحاضرة الثانية اساسيات الكم
... In 1913, Niels Bohr combined elements of quantum theory and classical physics in a treatment of the hydrogen atom. He stated two postulates for an electron in an atom: (i) Stationary states exist in which the energy of the electron is constant; such states ...
... In 1913, Niels Bohr combined elements of quantum theory and classical physics in a treatment of the hydrogen atom. He stated two postulates for an electron in an atom: (i) Stationary states exist in which the energy of the electron is constant; such states ...
Oct 24 Agenda
... Most of the atom’s mass and all of it’s positive charge are contained in a small core called the nucleus. Most of the volume of the atom is empty space through which the tiny negatively charged electrons are randomly dispersed. There are equal numbers of positive and negative charged particles so at ...
... Most of the atom’s mass and all of it’s positive charge are contained in a small core called the nucleus. Most of the volume of the atom is empty space through which the tiny negatively charged electrons are randomly dispersed. There are equal numbers of positive and negative charged particles so at ...
Modern Physics Important Concepts for AP Test
... o E = hf = (hc)/λ o p = E/c = h/ λ Matter equations (Matter does not move at c, do not use c = λּ f) o deBroglie Wavelength λ = h/p = h/(mv) (Common problem on exam) o f = E/h frequency of matter waves Davisson Germer Experiment measured wavelength of electrons. (wave properties of matter) o Fir ...
... o E = hf = (hc)/λ o p = E/c = h/ λ Matter equations (Matter does not move at c, do not use c = λּ f) o deBroglie Wavelength λ = h/p = h/(mv) (Common problem on exam) o f = E/h frequency of matter waves Davisson Germer Experiment measured wavelength of electrons. (wave properties of matter) o Fir ...
Set #4 - comsics
... mass, which lies halfway between them. (a) If such a system were a normal atom, how would its emission spectrum compared to that of hydrogen atom? (b) What would be the electron-positron separation, r, in the ground state orbit of positronium? ...
... mass, which lies halfway between them. (a) If such a system were a normal atom, how would its emission spectrum compared to that of hydrogen atom? (b) What would be the electron-positron separation, r, in the ground state orbit of positronium? ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.