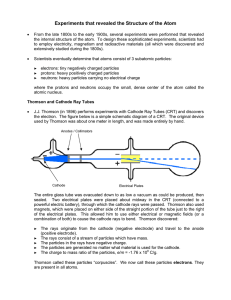
HW4 - SMU Physics
... electrodes. When a high-energy elementary particle passes through this space, it ionizes atoms of the gas. The strong electric field makes the resulting ions and electrons accelerate in opposite directions. They strike other atoms of the gas to ionize them, producing an avalanche of electrical disch ...
... electrodes. When a high-energy elementary particle passes through this space, it ionizes atoms of the gas. The strong electric field makes the resulting ions and electrons accelerate in opposite directions. They strike other atoms of the gas to ionize them, producing an avalanche of electrical disch ...
Pre-Knowledge: Chemistry and Physics Vocabulary Atomic Number
... The sum of the number of neutrons and protons in the nucleus of an atom. Nucleus The small “core” of the atom, where most of its mass and all of its positive charge is concentrated. Except for ordinary hydrogen (which has only a proton), atomic nuclei consist of protons and neutrons. For this reason ...
... The sum of the number of neutrons and protons in the nucleus of an atom. Nucleus The small “core” of the atom, where most of its mass and all of its positive charge is concentrated. Except for ordinary hydrogen (which has only a proton), atomic nuclei consist of protons and neutrons. For this reason ...
Atomic Structure
... elements can combine to form more than one compound C + O CO 1.00 g + 1.33 g 2.33 g ...
... elements can combine to form more than one compound C + O CO 1.00 g + 1.33 g 2.33 g ...
File - Mr. Gittermann
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
Chemical Change
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
Atomic Structure I. History A. prehistory The four elements B
... Matter is composed of extremely small particles called atoms ...
... Matter is composed of extremely small particles called atoms ...
Chapter 8: Chemical Reactions and Physical Changes
... • Protons: particle with a positive electrical charge ...
... • Protons: particle with a positive electrical charge ...
Atomic Structure Development
... Atomic Structure in the 1920s Worked with Rutherford 1910 – 1913 – returned to Oxford Bombarded various elements with electrons to produce X-rays. ...
... Atomic Structure in the 1920s Worked with Rutherford 1910 – 1913 – returned to Oxford Bombarded various elements with electrons to produce X-rays. ...
Atomic Structure (history of atom)
... ATOMS of any one ELEMENT are different from those of any other element Atoms of different elements can physically mix together or chemically combine to form compounds Chemical reactions occur when atoms are joined, separated or rearranged Atoms of one element are never changed into atoms of another ...
... ATOMS of any one ELEMENT are different from those of any other element Atoms of different elements can physically mix together or chemically combine to form compounds Chemical reactions occur when atoms are joined, separated or rearranged Atoms of one element are never changed into atoms of another ...
Learning Standards vocab chemical basis and molecules of life 09
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
Introduction to Chemistry
... To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffraction experiments were used to demonstrate the dual nature of all matter. To show t ...
... To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffraction experiments were used to demonstrate the dual nature of all matter. To show t ...
chapter2 2012 (no naming)
... This applies to all chemical reactions but DOES NOT include nuclear reactions ...
... This applies to all chemical reactions but DOES NOT include nuclear reactions ...
key - gcisd
... 6. Aristotle – everything was made from earth, wind, fire, water, and ether; alchemist 7. Democritus – first to propose the idea of an atom (indivisible particle)! 8. Dalton – developed the basis for the modern atomic theory ...
... 6. Aristotle – everything was made from earth, wind, fire, water, and ether; alchemist 7. Democritus – first to propose the idea of an atom (indivisible particle)! 8. Dalton – developed the basis for the modern atomic theory ...
2.1 The Nature of Matter - Sonoma Valley High School
... neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
... neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
Chap 1-3 Review
... A previously unknown element has the following information: Atomic number = 117 Atomic mass = 290 Describe this element in terms of number of each subatomic particle and predict the most likely ionic charge. ...
... A previously unknown element has the following information: Atomic number = 117 Atomic mass = 290 Describe this element in terms of number of each subatomic particle and predict the most likely ionic charge. ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.