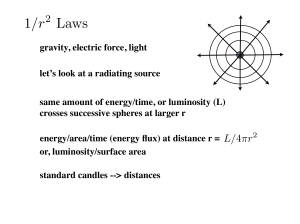
Lecture (2) - MIT OpenCourseWare
... Since the ratio of Δx is large the ratio of masses will be large : m(-) ___________ m(+) This negative particle from the cathode ray tubes was named the electron (m = 9.11 x 10 ...
... Since the ratio of Δx is large the ratio of masses will be large : m(-) ___________ m(+) This negative particle from the cathode ray tubes was named the electron (m = 9.11 x 10 ...
Bohr`s atomic model
... An orbiting electron must have a whole number of wavelengths around the circumference of its orbit (6), otherwise the wave will interfere destructively with itself. This approach predicts the same energy levels as Bohr’s model. (You can use Box 2 to show that Equations 1 and 2 are equivalent.) ...
... An orbiting electron must have a whole number of wavelengths around the circumference of its orbit (6), otherwise the wave will interfere destructively with itself. This approach predicts the same energy levels as Bohr’s model. (You can use Box 2 to show that Equations 1 and 2 are equivalent.) ...
Slide 1
... 4. When electrons in an atom in an excited state fall to lower energy levels, energy is 1. absorbed, only 2. released, only 3. neither released nor absorbed 4. both released and absorbed ...
... 4. When electrons in an atom in an excited state fall to lower energy levels, energy is 1. absorbed, only 2. released, only 3. neither released nor absorbed 4. both released and absorbed ...
Periodic Table
... Atomic Numbers • Atoms have no overall electrical charge so the number of __________ must equal the number of __________ . ...
... Atomic Numbers • Atoms have no overall electrical charge so the number of __________ must equal the number of __________ . ...
Matter Unit - OG
... 1.) Are made up of only one type of atom. 2) Cannot be broken down into any simpler substances by normal physical or chemical means. 3) Periodic Table of Elements *Familiarize yourself w/ it *Know what those numbers mean! ...
... 1.) Are made up of only one type of atom. 2) Cannot be broken down into any simpler substances by normal physical or chemical means. 3) Periodic Table of Elements *Familiarize yourself w/ it *Know what those numbers mean! ...
Basic Atomic Theory
... • The energy is therefore “quantized” – Only certain orbits with certain radii are possible – Orbits in between discrete value not possible ...
... • The energy is therefore “quantized” – Only certain orbits with certain radii are possible – Orbits in between discrete value not possible ...
The Quantum Mechanical Behavior of Light and Matter
... classical: matter behaves like particles, light behaves like waves quantum mechanics: both matter and light behave like both particles and waves ...
... classical: matter behaves like particles, light behaves like waves quantum mechanics: both matter and light behave like both particles and waves ...
Atomic Structure
... Daltons Atomic Theory • 1. All elements are composed of tiny indivisible particles called atoms. • 2. Atoms of the same element are identical. The atoms of one element are different from the atoms of another element. • 3. Atoms of different elements can physically mix together or can chemically com ...
... Daltons Atomic Theory • 1. All elements are composed of tiny indivisible particles called atoms. • 2. Atoms of the same element are identical. The atoms of one element are different from the atoms of another element. • 3. Atoms of different elements can physically mix together or can chemically com ...
Chapter 2: Matter
... Homogeneous: mixing occurs between the individual unit & is the same throughout. No ...
... Homogeneous: mixing occurs between the individual unit & is the same throughout. No ...
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER
... The spreading of waves around obstacles in their path. An example is the spreading of X-rays around regularly spaced atoms of a crystal; if the diffracted X-rays are directed onto a photographic film they provide information about the structure of the crystal. ...
... The spreading of waves around obstacles in their path. An example is the spreading of X-rays around regularly spaced atoms of a crystal; if the diffracted X-rays are directed onto a photographic film they provide information about the structure of the crystal. ...
MatterPP4
... energy level determines the chemical behavior of the different elements. Valence electrons are the outermost electrons in an atom. Elements with the same number of valence electrons have similar chemical properties. Group 1 has 1 valence electron, group 2 has 2, 13 has 3, 14 / 4, 15 / 5, 16 / 6, 17 ...
... energy level determines the chemical behavior of the different elements. Valence electrons are the outermost electrons in an atom. Elements with the same number of valence electrons have similar chemical properties. Group 1 has 1 valence electron, group 2 has 2, 13 has 3, 14 / 4, 15 / 5, 16 / 6, 17 ...
Development of the Model of the Atom
... photons. Because photons have the same energy as electrons, any attempt to locate them with a photon knocks the electron off its course. The Heisenburg Uncertainty Principle states that it is impossible to determining both the position and velocity of an electron at the same time. ...
... photons. Because photons have the same energy as electrons, any attempt to locate them with a photon knocks the electron off its course. The Heisenburg Uncertainty Principle states that it is impossible to determining both the position and velocity of an electron at the same time. ...
Pure Substances and Mixtures
... • Facts about atoms and elements: – Atoms are the basic building blocks of all matter (the smallest particle of matter) – There are different kinds of atoms. ...
... • Facts about atoms and elements: – Atoms are the basic building blocks of all matter (the smallest particle of matter) – There are different kinds of atoms. ...
Chapter 2: Data Analysis
... The light coming out of the excited atomic entities is very specific to ...
... The light coming out of the excited atomic entities is very specific to ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.