Electronic structure_(download)
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for the electrons in all the elements The extent to which it is real or simply an abstraction remains a fascinating, complex and unresolved argument ...
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for the electrons in all the elements The extent to which it is real or simply an abstraction remains a fascinating, complex and unresolved argument ...
Chapter 5 Electrons In Atoms 5.1 Models of the Atom The
... The quantum mechanical model description of how the ___________________ moving around the nucleus is similar to the motion of a rotating propeller blade. Atomic Orbitals An _______________________ orbital is often thought of as a region of space in which there is a high probability of finding an ele ...
... The quantum mechanical model description of how the ___________________ moving around the nucleus is similar to the motion of a rotating propeller blade. Atomic Orbitals An _______________________ orbital is often thought of as a region of space in which there is a high probability of finding an ele ...
Unit 2 Review KEY
... Electromagnetic Radiation – form of energy that exhibits wavelength behavior as it travels through space. Wavelength (λ) – the distance between corresponding points on adjacent waves. Frequency (v) – number of waves that pass a given point in a specific time (1 sec) Photoelectric Effect – an emissio ...
... Electromagnetic Radiation – form of energy that exhibits wavelength behavior as it travels through space. Wavelength (λ) – the distance between corresponding points on adjacent waves. Frequency (v) – number of waves that pass a given point in a specific time (1 sec) Photoelectric Effect – an emissio ...
Slide 1
... Energy quantization oPlanck oE=h. (h≈6.6260755x10-34 J.s) Photoelectric effect oWhen light strikes the surface of a metal and e- are ejected oE=h.c/ Bohr model oRadius of the circular orbits increase as n increases oAn atom with its e- in the lowest possible energy levels is said to be in its “ ...
... Energy quantization oPlanck oE=h. (h≈6.6260755x10-34 J.s) Photoelectric effect oWhen light strikes the surface of a metal and e- are ejected oE=h.c/ Bohr model oRadius of the circular orbits increase as n increases oAn atom with its e- in the lowest possible energy levels is said to be in its “ ...
The Address of the Electrons
... arrangement of electrons in an atom 3 methods of writing electron configuration ¡ Boxes ...
... arrangement of electrons in an atom 3 methods of writing electron configuration ¡ Boxes ...
Chapter 7 Lect. 2
... III. Orbital Shapes and Energies A. Atomic orbital shapes are surfaces that surround 90% of the total probability of where its electrons are 1. Look at l = 0, the s-orbitals 2. Basic shape of an s-orbital is spherical centered on the nucleus 3. Basic shape is same for same l values 4. Nodes = area ...
... III. Orbital Shapes and Energies A. Atomic orbital shapes are surfaces that surround 90% of the total probability of where its electrons are 1. Look at l = 0, the s-orbitals 2. Basic shape of an s-orbital is spherical centered on the nucleus 3. Basic shape is same for same l values 4. Nodes = area ...
Slide 1 - s3.amazonaws.com
... Chapter 7 Quantum Theory of the Atom 7.1 The Wave Nature of Light 7.2 Quantum Effects and Photons 7.3 The Bohr Theory of the Hydrogen Atom 7.4 Quantum Mechanics 7.5 Quantum Numbers and Atomic Orbitals ...
... Chapter 7 Quantum Theory of the Atom 7.1 The Wave Nature of Light 7.2 Quantum Effects and Photons 7.3 The Bohr Theory of the Hydrogen Atom 7.4 Quantum Mechanics 7.5 Quantum Numbers and Atomic Orbitals ...
Bohr Model, Quantum Mechanical Model
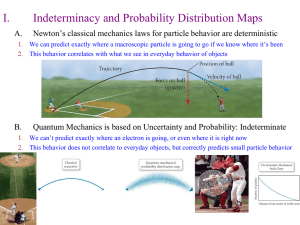
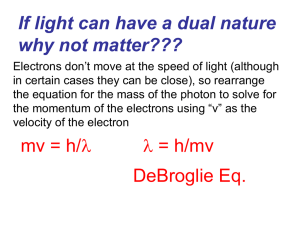
... b. energy is involved in moving an electron from one level to another. 4. Heisenberg Uncertainty Principle- It is impossible to know the momentum (mass of electron times velocity) of an electron and its position in space at the same time. One or the other. 5. Quantum Mechanical Model- a mathematical ...
... b. energy is involved in moving an electron from one level to another. 4. Heisenberg Uncertainty Principle- It is impossible to know the momentum (mass of electron times velocity) of an electron and its position in space at the same time. One or the other. 5. Quantum Mechanical Model- a mathematical ...
WAVE MECHANICS AND QUANTUM NUMBERS
... B. Quantum Theory- describes the wave properties of electrons in a mathematical fashion; it utilizes quantum numbers that describe the unique energy state of electrons. 1. Erwin Schrödinger 1926- used the new quantum theory to write and solve a mathematical equation describing the location and energ ...
... B. Quantum Theory- describes the wave properties of electrons in a mathematical fashion; it utilizes quantum numbers that describe the unique energy state of electrons. 1. Erwin Schrödinger 1926- used the new quantum theory to write and solve a mathematical equation describing the location and energ ...
Chapter 7 Student Learning Map
... photoelectric effect in describing the behavior of the electron and light? ...
... photoelectric effect in describing the behavior of the electron and light? ...
Answers to Critical Thinking Questions 4
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
Quantum Numbers Primer The quantum numbers
... Consider the hydrogen atom. When the electron occupies the n = 1 orbital (the lowest value allowed), the atom is at the lowest energy and the radius is the smallest. We call this lowest energy state the ground state. We can promote an electron in the n = 1 energy level to a higher level; such as n = ...
... Consider the hydrogen atom. When the electron occupies the n = 1 orbital (the lowest value allowed), the atom is at the lowest energy and the radius is the smallest. We call this lowest energy state the ground state. We can promote an electron in the n = 1 energy level to a higher level; such as n = ...
Section 5-2
... • Compare the Bohr and quantum mechanical models of the atom • Explain the impact of de Broglie’s waveparticle duality and the Heisenberg uncertainty principle on the modern view of electrons in atoms • Identify the relationships among a hydrogen atom’s energy levels, sublevels, and atomic ...
... • Compare the Bohr and quantum mechanical models of the atom • Explain the impact of de Broglie’s waveparticle duality and the Heisenberg uncertainty principle on the modern view of electrons in atoms • Identify the relationships among a hydrogen atom’s energy levels, sublevels, and atomic ...
Quantum Mechanical Model - Elmwood Park Memorial Middle School
... • Heisenberg Uncertainty Principleit is impossible to determine both the position and velocity of extremely small particles at the same time Why? Think about how particles are detected or how your eyes work… ...
... • Heisenberg Uncertainty Principleit is impossible to determine both the position and velocity of extremely small particles at the same time Why? Think about how particles are detected or how your eyes work… ...
Chapter 4 Arrangement of Electrons in Atoms
... • Explain how the Heisenberg uncertainty principle and the Schrödinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers and describe their significance. • Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per ...
... • Explain how the Heisenberg uncertainty principle and the Schrödinger wave equation led to the idea of atomic orbitals. • List the four quantum numbers and describe their significance. • Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per ...
Exam sample
... 7. “No two electrons in the same atom may have the same values for all four quantum numbers” is a statement of: a. Hund’s Rule. b. deBroglie’s Hypothesis. c. the Pauli Exclusion Principle. d. the Heisenberg Uncertainty Principle. 8. All s orbitals are: a. shaped like four-leaf clovers. b. dumbbell- ...
... 7. “No two electrons in the same atom may have the same values for all four quantum numbers” is a statement of: a. Hund’s Rule. b. deBroglie’s Hypothesis. c. the Pauli Exclusion Principle. d. the Heisenberg Uncertainty Principle. 8. All s orbitals are: a. shaped like four-leaf clovers. b. dumbbell- ...
Ch4 notes - Midway ISD
... • Photoelectric Effectelectrons are emitted from a metal’s surface when light of a certain frequency shines on the ...
... • Photoelectric Effectelectrons are emitted from a metal’s surface when light of a certain frequency shines on the ...
Exam and Study Notes
... o The Aufbau Principle (electrons start from the lowest energy) “The building up principle” The Aufbau Principle states that the to fill the 3d subshell, the 4s subshell must have 2 electrons in the subshell first o Pauli Exclusion Principle(Opposite spins) No two electron can have the same sp ...
... o The Aufbau Principle (electrons start from the lowest energy) “The building up principle” The Aufbau Principle states that the to fill the 3d subshell, the 4s subshell must have 2 electrons in the subshell first o Pauli Exclusion Principle(Opposite spins) No two electron can have the same sp ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.