Chapter 7
... A. Wave Nature of light Light is electromagnetic radiation. A type of energy embodies in oscillating electric and magnetic fields ...
... A. Wave Nature of light Light is electromagnetic radiation. A type of energy embodies in oscillating electric and magnetic fields ...
Chp7,Quantum_Num
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
mp2b-16 honors
... What was different about Planck's description of light compared to the previous theory of light? C. Know how to use the two equations of light - know the metric system! E=hx C=x D. What are the frequency, wavelength, and amplitude of a light wave? Be able to diagram. What happens when each is cha ...
... What was different about Planck's description of light compared to the previous theory of light? C. Know how to use the two equations of light - know the metric system! E=hx C=x D. What are the frequency, wavelength, and amplitude of a light wave? Be able to diagram. What happens when each is cha ...
eprint_11_28683_250
... position of the electron at the same instant in time. This is a statement of Heisenberg’s uncertainty principle. In order to get around this problem, rather than trying to define its exact position and momentum, we use the probability of finding the electron in a given volume of space. The probabil ...
... position of the electron at the same instant in time. This is a statement of Heisenberg’s uncertainty principle. In order to get around this problem, rather than trying to define its exact position and momentum, we use the probability of finding the electron in a given volume of space. The probabil ...
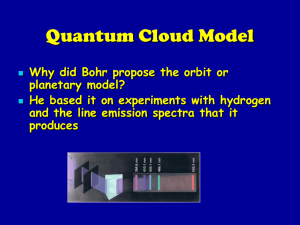
Quantum Cloud Model
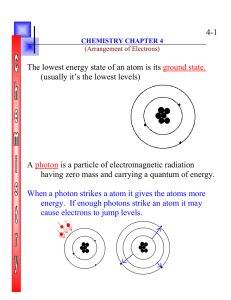
... The original location of the electron is referred to as the Ground State The higher level is called the Excited State When the electron returns to the ground state it gives off energy equal to the difference between the two levels (excited and ground state) as electromagnetic radiation with its spec ...
... The original location of the electron is referred to as the Ground State The higher level is called the Excited State When the electron returns to the ground state it gives off energy equal to the difference between the two levels (excited and ground state) as electromagnetic radiation with its spec ...
Chemistry 102 Summary June 25th - Bohr model only works for one
... Specific wave functions are called orbitals. Orbitals define the allowed energy states where electrons can reside. There are four basic shapes: s, p, d and f Shapes represent where an electron will reside 90 % of the time in that allowed energy state. From Heisenberg – the exact location cannot be d ...
... Specific wave functions are called orbitals. Orbitals define the allowed energy states where electrons can reside. There are four basic shapes: s, p, d and f Shapes represent where an electron will reside 90 % of the time in that allowed energy state. From Heisenberg – the exact location cannot be d ...
Powerpoint handout
... Bohr derived a more general formula to predict the observed energies of light: Each electron’s energy is determined by which level it is in. The levels are designated by whole numbers, n. ...
... Bohr derived a more general formula to predict the observed energies of light: Each electron’s energy is determined by which level it is in. The levels are designated by whole numbers, n. ...
l - Gordon State College
... What is the energy of one photon from a yellow light whose wavelength is 589 nm? 5.09 x 1014 Hz ...
... What is the energy of one photon from a yellow light whose wavelength is 589 nm? 5.09 x 1014 Hz ...
Which scientist developed the quantum mechanical model of the
... Which of the following states that no more than two electrons can occupy an atomic orbital and that two electrons in the same orbital must have opposite spins? A) B) C) D) ...
... Which of the following states that no more than two electrons can occupy an atomic orbital and that two electrons in the same orbital must have opposite spins? A) B) C) D) ...
apch07_quantum
... n=4 and l =3 is ____. e) The maximum number of orbitals that may be associated with the quantum number set n=3, l =2, and ml = -2 is ___. f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum ...
... n=4 and l =3 is ____. e) The maximum number of orbitals that may be associated with the quantum number set n=3, l =2, and ml = -2 is ___. f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum ...
Periodic Properties of the Elements
... of the orbital depends only on principle quantum number For atoms with more than 2 electrons, the energy depends on l & n ...
... of the orbital depends only on principle quantum number For atoms with more than 2 electrons, the energy depends on l & n ...
Document
... Chemistry 130 (Lecture VII-VIII) Answer 1. Which of the following statements is not consistent with a quantum mechanical view of nature? a. Matter can be thought of as waves b. Excited atoms can emit all possible energies c. Knowing the exact speed of an electron means we do not know anything about ...
... Chemistry 130 (Lecture VII-VIII) Answer 1. Which of the following statements is not consistent with a quantum mechanical view of nature? a. Matter can be thought of as waves b. Excited atoms can emit all possible energies c. Knowing the exact speed of an electron means we do not know anything about ...
Electron Configuration
... This involves looking at the most recent noble gas (since they are stable and all orbitals are full) and then continuing the electron configuration from there ...
... This involves looking at the most recent noble gas (since they are stable and all orbitals are full) and then continuing the electron configuration from there ...
4.quantumorbitals
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
Superconcepts
... 1. An atom’s bonding & reactive properties are determined by electron configuration. 2. The behavior of electrons in atoms is dictated by quantum mechanics. Concepts a. Newtonian physics don’t accurately describe the behavior of matter at the subatomic level. b. Both light and electrons behave as wa ...
... 1. An atom’s bonding & reactive properties are determined by electron configuration. 2. The behavior of electrons in atoms is dictated by quantum mechanics. Concepts a. Newtonian physics don’t accurately describe the behavior of matter at the subatomic level. b. Both light and electrons behave as wa ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.