energy levels
... – Tells you how far away the electron is from the nucleus – There are 1-7 energy levels, correlates with period numbers – Each level has same n number of sublevels – Maximum number of 2n2 electrons per level ...
... – Tells you how far away the electron is from the nucleus – There are 1-7 energy levels, correlates with period numbers – Each level has same n number of sublevels – Maximum number of 2n2 electrons per level ...
CH7 handout is here.
... 2. If the de Broglie wavelength of an electron is 15 nm, what is its velocity? The mass of an electron is 9.1 10-31 kg. ...
... 2. If the de Broglie wavelength of an electron is 15 nm, what is its velocity? The mass of an electron is 9.1 10-31 kg. ...
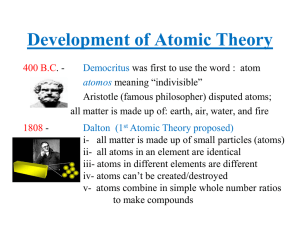
Development of Atomic Theory
... Heisenberg added to this concept that the position and velocity of an electron could never be simultaneously determined (Uncertainty Principle). All of these concepts/findings led to the development of our current model of the atom proposed by Schrodinger. ...
... Heisenberg added to this concept that the position and velocity of an electron could never be simultaneously determined (Uncertainty Principle). All of these concepts/findings led to the development of our current model of the atom proposed by Schrodinger. ...
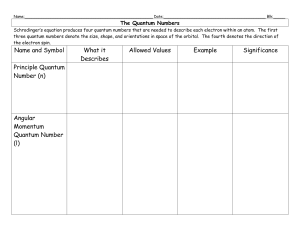
Quantum numbers
... The size of an atom The definition of the size of a hydrogen atom 1s orbital is the radius of the sphere that encloses 90% of the total electron probability. ...
... The size of an atom The definition of the size of a hydrogen atom 1s orbital is the radius of the sphere that encloses 90% of the total electron probability. ...
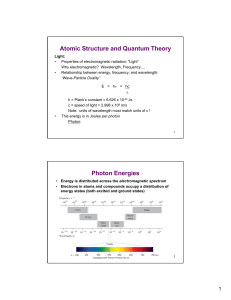
Atomic Structure and Quantum Theory Photon Energies
... Note: units of wavelength must match units of c ! This energy is in Joules per photon ...
... Note: units of wavelength must match units of c ! This energy is in Joules per photon ...
Quantum Numbers - Evan`s Chemistry Corner
... o ℓ =0 is called s o ℓ =1 is called p o ℓ =2 is called d o ℓ =3 is called f o For ℓ >3, the sublevels are named alphabetically, (g, h, and i), but there are no atoms with electrons in these locations. ...
... o ℓ =0 is called s o ℓ =1 is called p o ℓ =2 is called d o ℓ =3 is called f o For ℓ >3, the sublevels are named alphabetically, (g, h, and i), but there are no atoms with electrons in these locations. ...
Quantum Model of the Atom Power point
... •In order to describe orbitals, scientists use quantum numbers (specify the properties of atomic orbitals and the properties of electrons in orbitals). •The first three quantum numbers indicate the main energy level, the shape, and orientation of the orbital. •The fourth, the spin quantum number, de ...
... •In order to describe orbitals, scientists use quantum numbers (specify the properties of atomic orbitals and the properties of electrons in orbitals). •The first three quantum numbers indicate the main energy level, the shape, and orientation of the orbital. •The fourth, the spin quantum number, de ...
Modern Model of the Atom
... The most recent model of the atom is called the Quantum Mechanical Model. It was derived from a mathematical equation used to describe the energy and location of an electron in a hydrogen atom by the scientist, SHRODINGER. Characteristics of the model: ...
... The most recent model of the atom is called the Quantum Mechanical Model. It was derived from a mathematical equation used to describe the energy and location of an electron in a hydrogen atom by the scientist, SHRODINGER. Characteristics of the model: ...
1. Define the vocabulary on page 88. Section 1
... 3. All forms of electromagnetic radiation move at a constant speed of _____________ through a vacuum. 4. _________ is the distance between corresponding points on adjacent waves. 5. What is the symbol for wavelength? 6. Frequency is defined as _______________________________________. 7. What is the ...
... 3. All forms of electromagnetic radiation move at a constant speed of _____________ through a vacuum. 4. _________ is the distance between corresponding points on adjacent waves. 5. What is the symbol for wavelength? 6. Frequency is defined as _______________________________________. 7. What is the ...
Glossary Chapter 4
... created when the visible portion of light from excited atoms is shined through a prism (94) ...
... created when the visible portion of light from excited atoms is shined through a prism (94) ...
ELECTRONS IN ATOMS
... quantum mechanical model? __________________________________________________ same way as the motion of large objects.The quantum mechanical model is not based on the exact path an electron follows around the nucleus. 7. Is the following sentence true or false? The quantum mechanical model of the ato ...
... quantum mechanical model? __________________________________________________ same way as the motion of large objects.The quantum mechanical model is not based on the exact path an electron follows around the nucleus. 7. Is the following sentence true or false? The quantum mechanical model of the ato ...
quantum theory. Schrödinger equation
... atom is in an excited state when it has a higher potential energy than it has in its ground true 2 An state. Bohr's model of the atom, the orbit is the path taken by the atom’s protons around the false 3 In nucleus. Electrons theory is a scientific explanation for the fact that hydrogen atoms give o ...
... atom is in an excited state when it has a higher potential energy than it has in its ground true 2 An state. Bohr's model of the atom, the orbit is the path taken by the atom’s protons around the false 3 In nucleus. Electrons theory is a scientific explanation for the fact that hydrogen atoms give o ...
Particle-like Properties of Electromagnetic Radiation
... 3) Light energy can behave both as a wave and as small particles 4) atoms emit light quanta (photons) only of a few specific energies; this gives rise to the line spectrum (discussed in the previous lecture) ...
... 3) Light energy can behave both as a wave and as small particles 4) atoms emit light quanta (photons) only of a few specific energies; this gives rise to the line spectrum (discussed in the previous lecture) ...
No Slide Title
... emission spectrum that did not match known emission lines Mystery element was named Helium In 1895, William Ramsey discovered helium in a mineral of uranium (from alpha decay). ...
... emission spectrum that did not match known emission lines Mystery element was named Helium In 1895, William Ramsey discovered helium in a mineral of uranium (from alpha decay). ...
x 100 QUANTUM NUMBERS AND SYMBOLS
... 5. What type of orbital in an atom is designated by quantum numbers n=4, l =3, and ml =0? 6. A subshell in an atom has the values, n = 3, l =2. How many orbitals are there in this ...
... 5. What type of orbital in an atom is designated by quantum numbers n=4, l =3, and ml =0? 6. A subshell in an atom has the values, n = 3, l =2. How many orbitals are there in this ...
Chapter 7, Quantum Nos.
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
... Orbital Energies and Electron Configurations of Multi-Electron Atoms For the H atom the orbital energy depends only on n, so all orbitals with the same value of n have the same energy. This is not true, however, for any other atom! The H atom orbitals may be used to approximate the orbitals for mult ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.