Ch. 7 Sections 7.9 and 7.11 Powerpoint
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
... • There are many exceptions, but the most common ones are d4 and d9 For the purposes of this class, we are going to assume that ALL atoms (or ions) that end in d4 or d9 are exceptions to the rule. This may or may not be true, it just depends on the atom. ...
Unit 4 Study Guide - Key - Effingham County Schools
... electrons from metals that have absorbed photons. 11. In terms of energy, what must happen for an atom to change from the ground state to an excited state? _absorb energy________________________ 12. If an electron is at its lowest energy it is in the _ground_____________ state. 13. What is the Heise ...
... electrons from metals that have absorbed photons. 11. In terms of energy, what must happen for an atom to change from the ground state to an excited state? _absorb energy________________________ 12. If an electron is at its lowest energy it is in the _ground_____________ state. 13. What is the Heise ...
Chapter 5 Practice Section 5-1 Discuss the placement (if any) of
... If a quantum of energy is added to an atom, an electron ________________________________ ________________________________________. An ________________ is where you will most probably find an electron. How many orbitals are in the following sublevels? a. 4 p sublevel b. 3d sublevel How do the px, py, ...
... If a quantum of energy is added to an atom, an electron ________________________________ ________________________________________. An ________________ is where you will most probably find an electron. How many orbitals are in the following sublevels? a. 4 p sublevel b. 3d sublevel How do the px, py, ...
Ch 1 Lecture 2
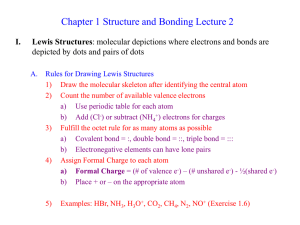
... 1) Draw the molecular skeleton after identifying the central atom 2) Count the number of available valence electrons a) Use periodic table for each atom b) Add (Cl-) or subtract (NH4+) electrons for charges 3) Fulfill the octet rule for as many atoms as possible a) Covalent bond = :, double bond = : ...
... 1) Draw the molecular skeleton after identifying the central atom 2) Count the number of available valence electrons a) Use periodic table for each atom b) Add (Cl-) or subtract (NH4+) electrons for charges 3) Fulfill the octet rule for as many atoms as possible a) Covalent bond = :, double bond = : ...
study note 1 06
... Use de Broglie's wave equation to explain why large objects do The huge m means a very small wavelength. not seem like waves Through a diffraction experiment. (showing destructive and constructive How can the wave properties of light be shown? interference) (as in fig. 6.16) Give an example showing ...
... Use de Broglie's wave equation to explain why large objects do The huge m means a very small wavelength. not seem like waves Through a diffraction experiment. (showing destructive and constructive How can the wave properties of light be shown? interference) (as in fig. 6.16) Give an example showing ...
Louie de Broglie
... However, the equation does not define the exact path the electron takes around the nucleus. It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. ...
... However, the equation does not define the exact path the electron takes around the nucleus. It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. ...
The Modern Atomic Model
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
... Bohr Model of the Atom (review) •Energy levels contain electrons. •Electrons travel around the nucleus. •Different orbitals varied by different quantum (energy). •Gaps between energy levels were not equal. ...
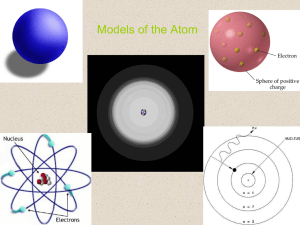
Models of the Atom
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
... Development of the Atomic Model • Could Rutherford’s atomic model explain the chemical properties of an element? No, to describe the chemical properties of an element we needed a model that better describes the behavior of electrons. ...
The Quantum Model of the Atom
... Principal Quantum Number Symbolized by n, indicates the main energy level occupied by the electron Positive values of 1,2,3,… As n increases, e- energy and distance from the nucleus increase n=1, first, or lowest, main energy level is closest to the nucleus Electrons with the same n value are ...
... Principal Quantum Number Symbolized by n, indicates the main energy level occupied by the electron Positive values of 1,2,3,… As n increases, e- energy and distance from the nucleus increase n=1, first, or lowest, main energy level is closest to the nucleus Electrons with the same n value are ...
Chapter 4-Arrangement of Electrons in Atoms
... -The atoms of each element have unique structures arising from interactions between electrons and the nucleus. -Atoms are so small that they are difficult to directly study. Atomic models are constructed to explain collections of experimental data. ...
... -The atoms of each element have unique structures arising from interactions between electrons and the nucleus. -Atoms are so small that they are difficult to directly study. Atomic models are constructed to explain collections of experimental data. ...
Precursors to Modern Physics
... atom is affected by large orbital quantum numbers? The state of an electron in an atom is completely defined by its quantum numbers. The energy of the electron is also a function of Z, the total positive charge of the nucleus. For the electrons with the same quantum numbers, what is the trend of the ...
... atom is affected by large orbital quantum numbers? The state of an electron in an atom is completely defined by its quantum numbers. The energy of the electron is also a function of Z, the total positive charge of the nucleus. For the electrons with the same quantum numbers, what is the trend of the ...
Section 4-2 The Quantum Model of the Atom Problems with the Bohr
... II. The only way to find an electron is with a photon III.Hitting an electron with a photon changes where the electron is IV. “It is impossible to determine simultaneously both the position and velocity of an electron or other subatomic particle” C. The Schrödinger Wave Equation I. This equation def ...
... II. The only way to find an electron is with a photon III.Hitting an electron with a photon changes where the electron is IV. “It is impossible to determine simultaneously both the position and velocity of an electron or other subatomic particle” C. The Schrödinger Wave Equation I. This equation def ...
Chapter 5 Worksheet 1
... The positions of very small objects like electrons can only be studied accurately by hitting them with very high energy (x-rays, gamma rays, etc.) and since the electron is so small it will have its momentum (motion) changed to a large extent by this high energy wave. 3. What is wave particle dualit ...
... The positions of very small objects like electrons can only be studied accurately by hitting them with very high energy (x-rays, gamma rays, etc.) and since the electron is so small it will have its momentum (motion) changed to a large extent by this high energy wave. 3. What is wave particle dualit ...
Quantum Mechanical Model of the Atom
... The Compton Effect: When a high energy xray photon collides with a “free electron”, it gives some of its energy to the electron and a lower energy photon scatters off the electron. ...
... The Compton Effect: When a high energy xray photon collides with a “free electron”, it gives some of its energy to the electron and a lower energy photon scatters off the electron. ...
Electrons-in
... • Niels Bohr – electrons are arranged in circular paths, or orbits, around the nucleus. – Does not account for unequal spacing of energy levels in an atom. ...
... • Niels Bohr – electrons are arranged in circular paths, or orbits, around the nucleus. – Does not account for unequal spacing of energy levels in an atom. ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.