Quantum Theory and Electrons as Waves
... If light could have particle-like behavior, then could matter have wave-like behavior? ...
... If light could have particle-like behavior, then could matter have wave-like behavior? ...
Atomic Orbitals and quantum numbers
... •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
... •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
Quantum Mechanical Model
... Directions: Complete the following notes and charts as you read through section 4.2 in your textbook. ...
... Directions: Complete the following notes and charts as you read through section 4.2 in your textbook. ...
AP Chemistry Chapter 6 Outline for Concepts to Know 6.1 Wave
... Concept of smallest unit of light energy as photon – having properties of both particles and waves Quantum as smallest possible packets or quantities of energy Photoelectric effect – effect of changing frequency? Changing intensity? Calculations using Planck’s constant, such as E=hv, will NO ...
... Concept of smallest unit of light energy as photon – having properties of both particles and waves Quantum as smallest possible packets or quantities of energy Photoelectric effect – effect of changing frequency? Changing intensity? Calculations using Planck’s constant, such as E=hv, will NO ...
7.4 The Wave Nature of Matter * 7.5 Quantum Mechanics and the Atom
... • Schrödinger's equation can be used derive the energies and orbitals of electrons in atoms Schrödinger equation ...
... • Schrödinger's equation can be used derive the energies and orbitals of electrons in atoms Schrödinger equation ...
Chapter 4 Test Question Topics
... 1- Know the definitions of the ground state and the excited states of an atom. 2- What must occur for an atom to move from the ground to the excited state or from the excited to the ground state? 3- Know the definitions of an electron cloud and an atomic nucleus. 4- What determines the size and shap ...
... 1- Know the definitions of the ground state and the excited states of an atom. 2- What must occur for an atom to move from the ground to the excited state or from the excited to the ground state? 3- Know the definitions of an electron cloud and an atomic nucleus. 4- What determines the size and shap ...
Aufbau Diagram Directions
... Pauli Exclusion: an atomic orbital may describe at most 2 electrons (each electron will have a different spin) Hund’s Rule: When electrons occupy orbitals of equal energy, one electron enters each orbital until all the orbitas contain one electron, then a second electron is added to each orbital. Ho ...
... Pauli Exclusion: an atomic orbital may describe at most 2 electrons (each electron will have a different spin) Hund’s Rule: When electrons occupy orbitals of equal energy, one electron enters each orbital until all the orbitas contain one electron, then a second electron is added to each orbital. Ho ...
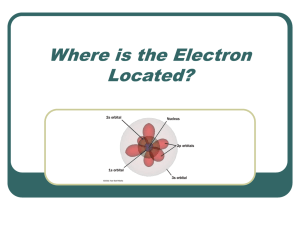
Where is the Electron Located?
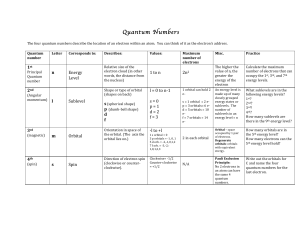
... Magnetic Quantum Number (m): Indicates the orientation of an orbital around the nucleus. Spin Quantum Number (↓↑): Indicates which way the electron is spinning ...
... Magnetic Quantum Number (m): Indicates the orientation of an orbital around the nucleus. Spin Quantum Number (↓↑): Indicates which way the electron is spinning ...
File - Chemistry 11 Enriched
... Each element has a specific electron configuration defining where the electrons are located. In order to understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroad ...
... Each element has a specific electron configuration defining where the electrons are located. In order to understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroad ...
The Quantum Mechanical Picture of the Atom
... The allowed energy states of atoms and molecules can be described by sets of numbers called quantum numbers ...
... The allowed energy states of atoms and molecules can be described by sets of numbers called quantum numbers ...
Electron configuration Jeopardy
... that. Line spectrum is certain colors show up in lines. You could pass electricity through an element for that. Who said that? 100 – He came up with the idea of quantum in 1900. Max Planck 200 – Electrons will make the maximum amount of unshared pairs possible. Hund 300 – The position and momentum o ...
... that. Line spectrum is certain colors show up in lines. You could pass electricity through an element for that. Who said that? 100 – He came up with the idea of quantum in 1900. Max Planck 200 – Electrons will make the maximum amount of unshared pairs possible. Hund 300 – The position and momentum o ...
The Quantum Mechanical Model of the Atom
... An atomic orbital can be visualized as a fuzzy cloud where the electron is most likely to be at a given energy level ...
... An atomic orbital can be visualized as a fuzzy cloud where the electron is most likely to be at a given energy level ...
Modern Model of the Atom Student Notes and Assignment
... The most recent model of the atom is called the Quantum Mechanical Model. It was derived from a mathematical equation used to describe the energy and location of an electron in a hydrogen atom by the scientist, SHRODINGER. Characteristics of the model: ...
... The most recent model of the atom is called the Quantum Mechanical Model. It was derived from a mathematical equation used to describe the energy and location of an electron in a hydrogen atom by the scientist, SHRODINGER. Characteristics of the model: ...
Electron Configuration and Chemical Periodicity
... used to describe the solutions (the orbitals are hydrogen-like) ...
... used to describe the solutions (the orbitals are hydrogen-like) ...
Chemistry 2000 Review: quantum mechanics of
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
... This equation was know to belong to a special class known as an eigenvector equation: an operator acts on a function (ψ) and generates a scalar times the same function Ψ is known as the wavefunction of the electron: there are an infinite number of such wavefunctions, each of which is characterized b ...
LT1: Electron.NOTES - Simpson County Schools
... What is the quantum mechanical model of the atom and how is it different from the Bohr model? _________________________________________________________________________________________________________ ____________________________________________________________________________________________________ ...
... What is the quantum mechanical model of the atom and how is it different from the Bohr model? _________________________________________________________________________________________________________ ____________________________________________________________________________________________________ ...
Chemistry I Unit Review: The Atom Text Chapters 2 and 7 1. The
... Determine the number of valence electrons and draw the dot diagram for the following atoms: ...
... Determine the number of valence electrons and draw the dot diagram for the following atoms: ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.